Maximum quantity allowed is 999
CAS RN: 4525-65-9 | Product Number: T3121
2,4,6-Trichlorophenyl Formate

Purity: >97.0%(HPLC)
- Formic Acid 2,4,6-Trichlorophenyl Ester
| Size | Unit Price | Same Day | 2-3 Business Days | Other Lead Time |
Shipping Information

|
|---|---|---|---|---|---|
| 1G |
NT$1,160
|
8 | 1 | Contact Us | |
| 5G |
NT$2,720
|
≥40 | ≥40 | Contact Us |
* The above prices include freight cost, customs, and other charges to the destination except for products that need to be shipped by sea or dry ice. For details, please contact
our distributor
in Taiwan to order our product.
* The storage conditions are subject to change without notice.
| Product Number | T3121 |
| Purity / Analysis Method | >97.0%(HPLC) |
| Molecular Formula / Molecular Weight | C__7H__3Cl__3O__2 = 225.45 |
| Physical State (20 deg.C) | Solid |
| Storage Temperature | Room Temperature (Recommended in a cool and dark place, <15°C) |
| Packaging and Container | 1G-Glass Bottle with Plastic Insert (View image) |
| CAS RN | 4525-65-9 |
| Reaxys Registry Number | 2524466 |
| PubChem Substance ID | 253661984 |
| Appearance | White to Almost white powder to crystal |
| Purity(HPLC) | min. 97.0 area% |
| Purity(Argentometric Titration) | min. 95.0 % |
| Melting point | 81.0 to 85.0 °C |
| Melting Point | 83 °C |
| Solubility (soluble in) | Toluene |
| H.S.code* | 2915.13-000 |

-
Used Chemicals
-
Procedure
-
A solution of 2-iodofluorene (500 mg, 1.71 mmol), 2,4,6-trichlorophenyl formate (579 mg, 2.57 mmol), palladium(II) acetate (38.4 mg, 0.171 mmol), Xantphos (198 mg, 0.342 mmol) and triethylamine (470 µL, 3.42 mmol) in toluene (10 mL) was stirred at room temperature for 24 hours. The solvent was removed under reduced pressure and the residue was washed with acetonitrile to give 1 as a pale yellow solid (462 mg, 69% yield).
A solution of 1 (460 mg, 1.18 mmol), triethylamine (330 µL, 2.36 mmol), DMAP (7.2 mg, 59 µmol) and morpholine (154 µL, 1.77 mmol) in THF (5 mL) was stirred at 45 °C for 20 hours. The reaction was quenched with water and the aqueous layer was extracted with ethyl acetate. The organic layer was dried over anhydrous sodium sulfate and filtered. The solvent was removed under reduced pressure and the residue was purified by silica gel column chromatography (ethyl acetate:hexane = 0:100 - 50:50) to give 2 as a white solid (305 mg, 93% yield).
-
Experimenter’s Comments
-
The reaction mixture at the first step was monitored by TLC (hexane:ethyl acetate = 1:19, Rf = 0.34).
The reaction mixture at the second step was monitored by TLC (hexane:ethyl acetate = 1:1, Rf = 0.26).
-
Analytical Data
-
2,4,6-Trichlorophenyl 9H-Fluorene-2-carboxylate (1)
(9H-Fluoren-2-yl)(morpholino)methanone (2)1H NMR (400 MHz, CDCl3);
(1) δ 8.41 (s, 1H), 8.29 (d, J = 8.1 Hz, 1H), 7.94-7.88 (m, 2H), 7.62 (d, J = 6.2 Hz, 1H), 7.48–7.38 (m, 4H), 4.02 (s, 2H).
(2) δ 7.81 (d, J = 7.6 Hz, 2H), 7.62-7.56 (m, 2H), 7.43-7.32 (m, 3H), 3.94 (s, 2H), 3.72 (brs, 8H). -
-
Lead Reference
-
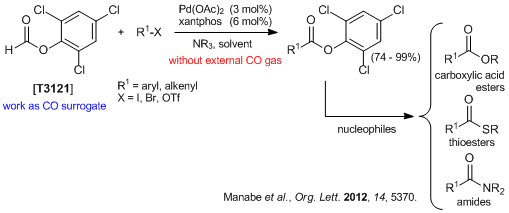
- Trichlorophenyl Formate: Highly Reactive and Easily Accessible Crystalline CO Surrogate for Palladium-Catalyzed Carbonylation of Aryl/Alkenyl Halides and Triflates.
-
Other References
-
- Pd-Catalyzed External-CO-Free Carbonylation: Preparation of 2,4,6-Trichlorophenyl 3,4-Dihydronaphthalene-2-Carboxylate
- Formic Acid Derivatives as Practical Carbon Monoxide Surrogates for Metal-Catalyzed Carbonylation Reactions
References
- 1)T. Ueda, H. Konishi, K. Manabe, Org. Lett. 2012, 14, 5370.

- 2)H. Konishi, T. Ueda, K. Manabe, Org. Synth. 2014, 91, 39.

- 3)H. Konishi, K. Manabe, Synlett 2014, 25, 1971.

Articles/Brochures
[TCI Practical Example] Introduction of Carbonyl Group by Using 2,4,6-Trichlorophenyl Formate
Safety Data Sheet (SDS)
The requested SDS is not available.
Please Contact Us for more information.
Specifications
C of A & Other Certificates
Sample C of A
A sample C of A for this product is not available at this time.
Analytical Charts
The requested analytical chart is not available. Sorry for the inconvenience.



