Maximum quantity allowed is 999
Please select the quantity
CAS RN: 4136-95-2 | Product Number: T1413
2,4,6-Trichlorobenzoyl Chloride

Purity: >98.0%(GC)(T)
Synonyms:
Product Documents:
| Size | Unit Price | Same Day | 2-3 Business Days | Other Lead Time |
Shipping Information

|
|---|---|---|---|---|---|
| 5G |
NT$3,560
|
6 | 0 | Contact Us | |
| 25G |
NT$12,320
|
30 | 6 | Contact Us |
* The above prices include freight cost, customs, and other charges to the destination except for products that need to be shipped by sea or dry ice. For details, please contact
our distributor
in Taiwan to order our product.
* The storage conditions are subject to change without notice.
| Product Number | T1413 |
| Purity / Analysis Method | >98.0%(GC)(T) |
| Molecular Formula / Molecular Weight | C__7H__2Cl__4O = 243.89 |
| Physical State (20 deg.C) | Liquid |
| Storage Temperature | Room Temperature (Recommended in a cool and dark place, <15°C) |
| Store Under Inert Gas | Store under inert gas |
| Condition to Avoid | Moisture Sensitive |
| CAS RN | 4136-95-2 |
| Reaxys Registry Number | 2050280 |
| PubChem Substance ID | 87577179 |
| MDL Number | MFCD00075323 |
Specifications
| Appearance | Colorless to Light yellow clear liquid |
| Purity(GC) | min. 98.0 % |
| Purity(Argentometric Titration) | min. 98.0 % |
Properties (reference)
| Boiling Point | 275 °C |
| Specific Gravity (20/20) | 1.56 |
| Refractive Index | 1.58 |
| Solubility (soluble in) | Toluene |
GHS
| Pictogram |

|
| Signal Word | Danger |
| Hazard Statements | H314 : Causes severe skin burns and eye damage. H290 : May be corrosive to metals. |
| Precautionary Statements | P501 : Dispose of contents/ container to an approved waste disposal plant. P234 : Keep only in original container. P264 : Wash skin thoroughly after handling. P280 : Wear protective gloves/ protective clothing/ eye protection/ face protection. P390 : Absorb spillage to prevent material damage. P303 + P361 + P353 : IF ON SKIN (or hair): Take off immediately all contaminated clothing. Rinse skin with water/shower. P301 + P330 + P331 : IF SWALLOWED: Rinse mouth. Do NOT induce vomiting. P363 : Wash contaminated clothing before reuse. P304 + P340 + P310 : IF INHALED: Remove person to fresh air and keep comfortable for breathing. Immediately call a POISON CENTER/doctor. P305 + P351 + P338 + P310 : IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. Immediately call a POISON CENTER/doctor. P406 : Store in corrosive resistant container with a resistant inner liner. P405 : Store locked up. |
Related Laws:
Transport Information:
| UN Number | UN3265 |
| Class | 8 |
| Packing Group | II |
| H.S.code* | 2916.39-000 |
Application
Yamaguchi Esterification and Macrolactonization
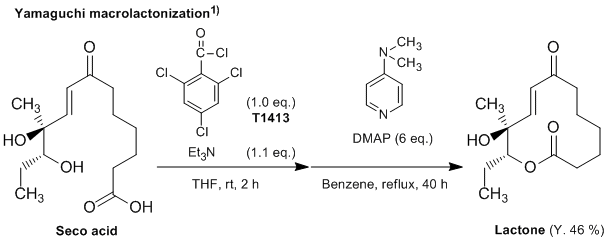
2,4,6-Trichlorobenzoyl chloride is often used for the Yamaguchi esterification and macrolactonization. The Yamaguchi esterification is the rapid and mild esterification method in the presence of 4-dimethylaminopyridine (DMAP) using a mixed anhydride, which is prepared from 2,4,6-trichlorobenzoyl chloride and the carboxylic acid. 1–3) The features of this esterification are as follows: 1) the reaction is conducted under high-dilution conditions to minimize intermolecular coupling; 2) the mixed anhydride is dissolved and slowly added to a refluxing solution of DMAP in aromatic hydrocarbons (e.g. benzene, toluene); 3) usually several equivalents of DMAP, as a catalyst for acylation, are used. It is especially useful in the synthesis of macrolactones with intramolecular esterification, called the Yamaguchi macrolactonization. 1,4)
Typical procedure1): A mixture of the seco acid (272 mg) and triethylamine (153 µL) in THF (10 mL) is stirred for 10 min at room temperature, and then 2,4,6-trichlorobenzoyl chloride (160 µL) is added. After stirring for 2 h at room temperature, the resulting precipitate is filtered and washed with a small amount of THF. The filtrate is diluted with benzene (500 mL) and slowly added to a refluxing solution of DMAP (732 mg) in benzene (100 mL) over a period of 40 h. The reaction mixture is washed successively with a saturated aqueous citric acid solution, water, an aqueous sodium hydrogen carbonate, and water, and dried over anhydrous magnesium sulfate. Concentration of the filtrate under reduced pressure affords a crude product. The product is separated by preparative TLC (eluent: diethyl ether / benzene = 2 / 1) on silica gel to give 116 mg of the lactone (46 % yield).
References
- 1)Seminal Publications
- 2)Reviews
- 3)Theoretical Studies
- 4)Stereoselective Synthesis of Macrolides
- a)M. Hikota, H. Tone, K. Horita O. Yonemitsu, Tetrahedron 1990, 46, 4613.

- b)A. K. Ghosh, Y. Wang, J. T. Kim, J. Org. Chem. 2001,66, 8973.

- c)J. P. Marino, M. S. McClure, D. P. Holub, J. V. Comasseto, F. C. Tucci, J. Am. Chem. Soc., 2002, 124, 1664.

- d)T. Hu, N. Takenaka, J. S. Panek, J. Am. Chem. Soc., 2002, 124, 12806.

- a)M. Hikota, H. Tone, K. Horita O. Yonemitsu, Tetrahedron 1990, 46, 4613.
PubMed Literature
Articles/Brochures
TCIMAIL
Product Documents (Note: Some products will not have analytical charts available.)
Safety Data Sheet (SDS)
Please select Language.
The requested SDS is not available.
Please Contact Us for more information.
Specifications
C of A & Other Certificates
Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
Sample C of A
This is a sample C of A and may not represent a recently manufactured lot of the product.
A sample C of A for this product is not available at this time.
Analytical Charts
Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
The requested analytical chart is not available. Sorry for the inconvenience.



