Maximum quantity allowed is 999
Please select the quantity
CAS RN: 34946-82-2 | Product Number: T1292
Copper(II) Trifluoromethanesulfonate

Purity: >98.0%(T)
Synonyms:
- Copper(II) Triflate
- Trifluoromethanesulfonic Acid Copper(II) Salt
Product Documents:
| Size | Unit Price | Same Day | 2-3 Business Days |
|---|---|---|---|
| 5G |
NT$2,280
|
10 | ≥100 |
| 25G |
NT$6,960
|
11 | 34 |
* The above prices include freight cost, customs, and other charges to the destination except for products that need to be shipped by sea or dry ice. For details, please contact
our distributor
in Taiwan to order our product.
* The storage conditions are subject to change without notice.
| Product Number | T1292 |
| Purity / Analysis Method | >98.0%(T) |
| Molecular Formula / Molecular Weight | C__2CuF__6O__6S__2 = 361.67 |
| Physical State (20 deg.C) | Solid |
| Storage Temperature | Room Temperature (Recommended in a cool and dark place, <15°C) |
| Store Under Inert Gas | Store under inert gas |
| Condition to Avoid | Hygroscopic |
| CAS RN | 34946-82-2 |
| Reaxys Registry Number | 4028198 |
| PubChem Substance ID | 87577063 |
| MDL Number | MFCD00077492 |
Specifications
| Appearance | White to Gray to Dark blue powder to crystal |
| Purity(Chelometric Titration) | min. 98.0 % |
Properties (reference)
| Solubility in water | Soluble |
GHS
| Pictogram |

|
| Signal Word | Danger |
| Hazard Statements | H314 : Causes severe skin burns and eye damage. |
| Precautionary Statements | P501 : Dispose of contents/ container to an approved waste disposal plant. P260 : Do not breathe dusts or mists. P264 : Wash skin thoroughly after handling. P280 : Wear protective gloves/ protective clothing/ eye protection/ face protection. P303 + P361 + P353 : IF ON SKIN (or hair): Take off immediately all contaminated clothing. Rinse skin with water/shower. P301 + P330 + P331 : IF SWALLOWED: Rinse mouth. Do NOT induce vomiting. P363 : Wash contaminated clothing before reuse. P304 + P340 + P310 : IF INHALED: Remove person to fresh air and keep comfortable for breathing. Immediately call a POISON CENTER/doctor. P305 + P351 + P338 + P310 : IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. Immediately call a POISON CENTER/doctor. P405 : Store locked up. |
Related Laws:
Transport Information:
| UN Number | UN1759 |
| Class | 8 |
| Packing Group | III |
| H.S.code* | 2904.10-000 |
Application
Copper (II) Triflate Catalyzed Regioselective Markovnikov Hydration of Alkynes

Typical Procedure (R1 = 4-methoxybenznene, R2 = H): Cu(OTf)2 (72 mg, 20 mol%) and water (36 mg, 2 eq.) are added to a stirred solution of 1-ethynyl-4-methoxybenzene (132.2 mg, 1 mmol) in EtOAc (2 mL). The reaction mixture is stirred at 100 °C for 1 hr. After completion of the reaction, it is cooled to room temperature; water is added and extracted with EtOAc, dried over anhydrous Na2SO4 and concentrated. The residue is purified by column chromatography to give 4'-methoxyacetophenone (135 mg, 90%) as a white solid.
References
Application
Synthesis of Optically Active Oxazoline Derivatives via Asymmetric Desymmetrization of 1,3-Diols
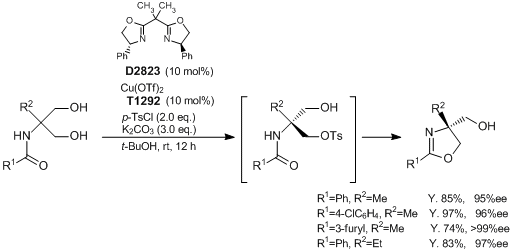
Typical Procedure:
Amino diols (0.5 mmol), K2CO3 (207.3 mg, 1.5 mmol), and p-toluenesulfonyl chloride (190.7 mg, 1.0 mmol) are added to a solution of Cu(OTf)2 (18.1 mg, 0.05 mmol) and (R,R)-2,2'-isopropylidenebis(4-phenyl-2-oxazoline) (16.7 mg, 0.05 mmol) in tert-butyl alcohol (2 mL). After stirring for 12 h at room temperature, water (10 mL) is added. The resulting mixture is extracted with AcOEt. After separation, the organic layer is washed with brine, and then dried over Na2SO4. Concentration and purification through flash column chromatography gives the product.
Amino diols (0.5 mmol), K2CO3 (207.3 mg, 1.5 mmol), and p-toluenesulfonyl chloride (190.7 mg, 1.0 mmol) are added to a solution of Cu(OTf)2 (18.1 mg, 0.05 mmol) and (R,R)-2,2'-isopropylidenebis(4-phenyl-2-oxazoline) (16.7 mg, 0.05 mmol) in tert-butyl alcohol (2 mL). After stirring for 12 h at room temperature, water (10 mL) is added. The resulting mixture is extracted with AcOEt. After separation, the organic layer is washed with brine, and then dried over Na2SO4. Concentration and purification through flash column chromatography gives the product.
References
Application
Efficient preparation method for alpha, omega-amino alcohols

Reference
PubMed Literature
Articles/Brochures
TCIMAIL
[Research Articles] Copper (II) Triflate Catalyzed Regioselective Markovnikov Hydration of Alkynes[Research Articles] Synthesis of Optically Active Oxazoline Derivatives via Asymmetric Desymmetrization of 1,3-Diols
Product Documents (Note: Some products will not have analytical charts available.)
Safety Data Sheet (SDS)
Please select Language.
The requested SDS is not available.
Please Contact Us for more information.
Specifications
C of A & Other Certificates
Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
Sample C of A
This is a sample C of A and may not represent a recently manufactured lot of the product.
A sample C of A for this product is not available at this time.
Analytical Charts
Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
The requested analytical chart is not available. Sorry for the inconvenience.



