Maximum quantity allowed is 999
Please select the quantity
CAS RN: 415918-91-1 | Product Number: D4994
(R,R,R)-(3,5-Dioxa-4-phosphacyclohepta[2,1-a:3,4-a']dinaphthalen-4-yl)bis(1-phenylethyl)amine
![(R,R,R)-(3,5-Dioxa-4-phosphacyclohepta[2,1-a:3,4-a']dinaphthalen-4-yl)bis(1-phenylethyl)amine No-Image](/medias/D4994.jpg?context=bWFzdGVyfHJvb3R8Njc4OTd8aW1hZ2UvanBlZ3xhRFJoTDJneE5DODRPVEl4TkRZMU1EazBNVGMwTDBRME9UazBMbXB3Wnd8MzUxYTFkOTQ0M2I2ODk2MzQxNWY5ZjUxNmI1NDlhYTNkZmZmZmVlYzBlYWE1NjdmNjdlYjUyY2MxOWE2Mzk5Zg)
Purity: >98.0%(HPLC)
Synonyms:
- (11bR)-N,N-Bis[(1R)-1-phenylethyl]dinaphtho[2,1-d:1',2'-f][1,3,2]dioxaphosphepin-4-amine
Product Documents:
| Size | Unit Price | Same Day | 2-3 Business Days | Other Lead Time |
Shipping Information

|
|---|---|---|---|---|---|
| 200MG |
NT$5,680
|
6 | ≥60 | Contact Us | |
| 1G |
NT$17,920
|
1 | 0 | Contact Us |
* The above prices include freight cost, customs, and other charges to the destination except for products that need to be shipped by sea or dry ice. For details, please contact
our distributor
in Taiwan to order our product.
* The storage conditions are subject to change without notice.
| Product Number | D4994 |
| Purity / Analysis Method | >98.0%(HPLC) |
| Molecular Formula / Molecular Weight | C__3__6H__3__0NO__2P = 539.61 |
| Physical State (20 deg.C) | Solid |
| Storage Temperature | Room Temperature (Recommended in a cool and dark place, <15°C) |
| Store Under Inert Gas | Store under inert gas |
| Condition to Avoid | Air Sensitive |
| Packaging and Container | 1G-Glass Bottle with Plastic Insert (View image), 200MG-Glass Bottle with Plastic Insert (View image) |
| CAS RN | 415918-91-1 |
| PubChem Substance ID | 354334571 |
| MDL Number | MFCD08561138 |
Specifications
| Appearance | White to Light yellow powder to crystal |
| Purity(HPLC) | min. 95.0 area% |
| Optical purity(LC) | min. 98.0 ee% |
| Elemental analysis(Nitrogen) | 2.20 to 3.00 % |
Properties (reference)
GHS
Related Laws:
Transport Information:
| H.S.code* | 2934.99-000 |
Application
Iridium / Axially Chiral Phosphoramidite Complex Catalyzed Allylic Substitution of Allyl Carbonate Derivatives
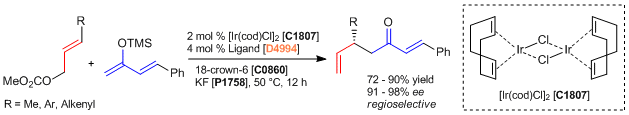
In a nitrogen-filled atmosphere, [Ir(cod)Cl]2 (2.7 mg, 0.0040 mmol), the ligand (4.3 mg, 0.0080 mmol), KF (12 mg, 0.20 mmol), 18-crown-6 (53 mg, 0.20 mmol) and a Teflon-coated magnetic stirring bar are added to a 1-dram vial. Then, anhydrous THF (0.40 mL) is added. The mixture is stirred for 5 min. at ambient temperature before allyl carbonate derivative (0.20 mmol) and the silyl enolate derivative (0.40 mmol, 2.0 equiv) are added. The vial is sealed with a cap and stirred at 50 °C for 12 h. Then, water (1 mL) is added to the reaction mixture followed by 1 N HCl (0.5 mL). The resulting mixture is stirred vigorously for 3 h at room temperature. Brine (1 mL) and Et2O (1 mL) are added; the organic layer is separated, and the aqueous layer is extracted with Et2O (3 x 3 mL). The combined organic extracts are dried over anhydrous magnesium sulfate, filtered, and concentrated under reduced pressure. Purification of the crude product is performed by flash column chromatography (gradient elution: hexane:Et2O = 50:1 to 5:1) to provide the product.
References
- Iridium-Catalyzed Enantioselective Allylic Substitution of Unstabilized Enolates Derived from α,β-Unsaturated Ketones
PubMed Literature
Articles/Brochures
TCIMAIL
Product Documents (Note: Some products will not have analytical charts available.)
Safety Data Sheet (SDS)
Please select Language.
The requested SDS is not available.
Please Contact Us for more information.
Specifications
C of A & Other Certificates
Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
Sample C of A
This is a sample C of A and may not represent a recently manufactured lot of the product.
A sample C of A for this product is not available at this time.
Analytical Charts
Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
The requested analytical chart is not available. Sorry for the inconvenience.

![(R,R,R)-(3,5-Dioxa-4-phosphacyclohepta[2,1-a:3,4-a']dinaphthalen-4-yl)bis(1-phenylethyl)amine (R,R,R)-(3,5-Dioxa-4-phosphacyclohepta[2,1-a:3,4-a']dinaphthalen-4-yl)bis(1-phenylethyl)amine](/_ui/responsive/theme-tci/images/missing_product_zoom.jpg)

