Maximum quantity allowed is 999
Please select the quantity
CAS RN: 50-91-9 | Product Number: D2235
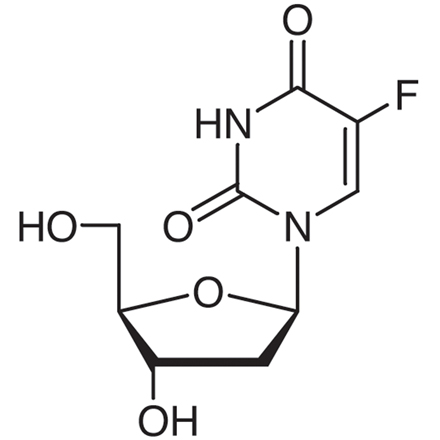
2'-Deoxy-5-fluorouridine
Purity: >98.0%(T)(HPLC)
Synonyms:
- 1-(2-Deoxy-β-D-ribofuranosyl)-5-fluorouracil
- 5-Fluoro-2'-deoxy-β-uridine
- Floxuridine
- FUDR
Product Documents:
* The above prices include freight cost, customs, and other charges to the destination except for products that need to be shipped by sea or dry ice. For details, please contact
our distributor
in Taiwan to order our product.
* The storage conditions are subject to change without notice.
| Product Number | D2235 |
| Purity / Analysis Method | >98.0%(T)(HPLC) |
| Molecular Formula / Molecular Weight | C__9H__1__1FN__2O__5 = 246.19 |
| Physical State (20 deg.C) | Solid |
| Storage Temperature | Room Temperature (Recommended in a cool and dark place, <15°C) |
| Packaging and Container | 100MG-Glass Bottle with Plastic Insert (View image), 1G-Glass Bottle with Plastic Insert (View image), 500MG-Glass Bottle with Plastic Insert (View image) |
| CAS RN | 50-91-9 |
| Reaxys Registry Number | 90221 |
| PubChem Substance ID | 87568673 |
| SDBS (AIST Spectral DB) | 19323 |
| Merck Index (14) | 4112 |
| MDL Number | MFCD00006530 |
Specifications
| Appearance | White to Almost white powder to crystal |
| Purity(HPLC) | min. 98.0 area% |
| Melting point | 148.0 to 152.0 °C |
Properties (reference)
| Melting Point | 150 °C |
| Specific Rotation | 38° (C=1,H2O) |
| Solubility in water | Soluble |
| Solubility (soluble in) | Alcohol, Acetone |
| Solubility (insoluble in) | Ether, Benzene, Chloroform |
GHS
| Pictogram |

|
| Signal Word | Danger |
| Hazard Statements | H301 : Toxic if swallowed. |
| Precautionary Statements | P501 : Dispose of contents/ container to an approved waste disposal plant. P270 : Do not eat, drink or smoke when using this product. P264 : Wash skin thoroughly after handling. P301 + P310 + P330 : IF SWALLOWED: Immediately call a POISON CENTER/doctor. Rinse mouth. P405 : Store locked up. |
Related Laws:
| RTECS# | YU7525000 |
Transport Information:
| UN Number | UN2811 |
| Class | 6.1 |
| Packing Group | III |
| H.S.code* | 2934.99-000 |
Application
Floxuridine: An Antitumor Antimetabolite with Inhibition of Thymidylate Synthase
Floxuridine (FUDR), an antitumor antimetabolite, is a prodrug of 5-fluorouracil (5-FU) [F0151]. Floxuridine is rapidly catabolized to 5-FU in vivo. Similarly to 5-FU, floxuridine inhibits thymidilate synthetase (TS) which is an enzyme that catalyzes the conversion of deoxyuridine monophosphate (dUMP) to deoxythymidine monophosphate (dTMP). dTMP forms thymidine, a nucleic acid in deoxyribonucleic acid (DNA). Therefore, the primary effect of floxuridine is to interfere with the synthesis of DNA. (The product is for research purpose only.)
References
- Mechanism of induction of gastrointestinal toxicity in the mouse by 5-fluorouracil, 5-fluorouridine, and 5-fluoro-2'-deoxyuridine
- Thymidylate synthase inhibitors in cancer therapy: direct and indirect inhibitors (a review)
- Comparison of 5-fluoro-2'-deoxyuridine with 5-fluorouracil and their role in the treatment of colorectal cancer (a review)
PubMed Literature
Articles/Brochures
TCIMAIL
Product Documents (Note: Some products will not have analytical charts available.)
Safety Data Sheet (SDS)
Please select Language.
The requested SDS is not available.
Please Contact Us for more information.
Specifications
C of A & Other Certificates
Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
Sample C of A
This is a sample C of A and may not represent a recently manufactured lot of the product.
A sample C of A for this product is not available at this time.
Analytical Charts
Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
The requested analytical chart is not available. Sorry for the inconvenience.




