Maximum quantity allowed is 999
Please select the quantity
Imidazolidinone Derivatives for Versatile Asymmetric Reactions
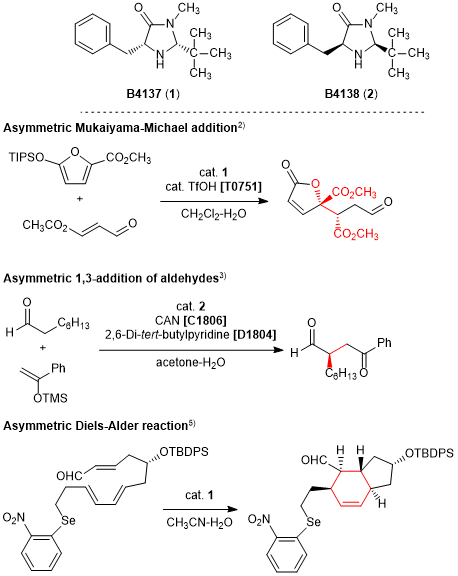
The imidazolidinone derivatives (1,2) are asymmetric organocatalysts developed by MacMillan et al. To date, various types of asymmetric reactions using 1 and 2 have been reported, such as Mukaiyama-Michael addition, epoxidation of α,β-unsaturated aldehydes, 1,3-addition of aldehydes, and the Diels-Alder reaction. The desired products are generated in high yields and selectivities in all cases. These reactions are often utilized in the total syntheses of natural products to construct complex condensed-ring structures such as in spinosyn. Thus, 1 and 2 are powerful tools and their use in new asymmetric reactions is anticipated.
Related Products
- B4137
- (2R,5R)-(+)-2-tert-Butyl-3-methyl-5-benzyl-4-imidazolidinone
- B4138
- (2S,5S)-(-)-2-tert-Butyl-3-methyl-5-benzyl-4-imidazolidinone
References
- 1)The First Enantioselective Organocatalytic Mukaiyama-Michael Reaction: A Direct Method for the Synthesis of Enantioenriched γ-Butenolide Architecture
- 2)Enantioselective organocatalytic epoxidation using hypervalent iodine reagents
- 3)Enantioselective Organocatalytic Singly Occupied Molecular Orbital Activation: The Enantioselective α-Enolation of Aldehydes
- 4)Enantioselective Organocatalytic Intramolecular Diels−Alder Reactions. The Asymmetric Synthesis of Solanapyrone D
- 5)Total Synthesis of (−)-Spinosyn A via Carbonylative Macrolactonization