Maximum quantity allowed is 999
CAS RN: 2833773-70-7 | 产品编码: T4082
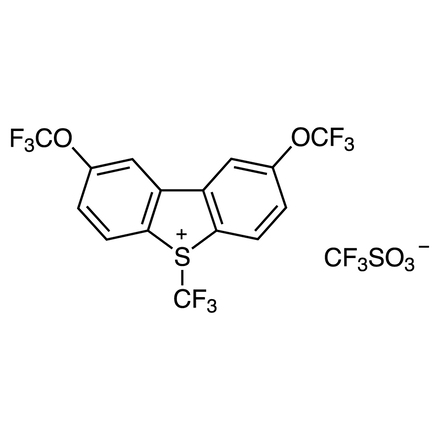
S-Trifluoromethyl-2,8-bis(trifluoromethoxy)dibenzothiophenium Triflate
| 产品编码 | T4082 |
| 纯度/分析方法 | >98.0%(T)(HPLC) |
| 分子式/分子量 | C__1__6H__6F__1__2O__5S__2 = 570.32 |
| 外观与形状(20°C) | 固体 |
| 储存温度 | 室温 (15°C以下阴凉干燥处) |
| 储存在惰性气体下 | 存放于惰性气体之中 |
| 应避免的情况 | 湿气 (吸湿) |
| 包装和容器 | 1G-Glass Bottle with Plastic Insert (查看图片) |
| CAS RN | 2833773-70-7 |
| Reaxys-RN | 43802338 |
| Appearance | White to Light yellow powder to crystal |
| Purity(HPLC) | min. 98.0 area% |
| Purity(Neutralization titration) | min. 98.0 % |
| Melting point | 152.0 to 156.0 °C |
| NMR | confirm to structure |
| 熔点 | 154 °C |
| 象形图 |

|
| 信号词 | Warning |
| 危险性说明 | H315 : Causes skin irritation. H319 : Causes serious eye irritation. |
| 防范说明 | P264 : Wash skin thoroughly after handling. P280 : Wear protective gloves/ eye protection/ face protection. P302 + P352 : IF ON SKIN: Wash with plenty of water. P337 + P313 : If eye irritation persists: Get medical advice/ attention. P305 + P351 + P338 : IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. P362 + P364 : Take off contaminated clothing and wash it before reuse. P332 + P313 : If skin irritation occurs: Get medical advice/ attention. |
| HS编码* | 2934.99-000 |

-
Used Chemicals
-
Procedure
-
Sodium hydride (60%, 76.1 mg, 3.17 mmol) was adde to a solution of α-acetyl-γ-butyrolactone (200 mg, 1.59 mmol) in DMF (4 mL) at room temperature for 15 minutes. The reaction mixture was cooled to -45 °C and Umemoto reagent IV (1.08 g, 1.90 mmol) was added. Then the mixture was allowed to warm to room temperature and was stirred for 1 hour. The reaction was quenched with water and the aqueous layer was extracted with ethyl acetate. The organic layer was dried over anhydrous sodium sulfate and filtered. The solvent was removed under reduced pressure and the residue was purified by silica gel column chromatography (ethyl acetate:hexane = 0:100 - 20:80) to give 3-acetyl-3-(trifluoromethyl)dihydrofuran-2(3H)-one as a yellow oil (218 mg, 71% yield).
-
Experimenter’s Comments
-
The reaction mixture was monitored by NMR.
-
Analytical Data
-
3-Acetyl-3-(trifluoromethyl)dihydrofuran-2(3H)-one
1H NMR (270 MHz, CDCl3); δ 4.45-4.37 (m,1H), 4.31-4.22 (m, 1H), 3.07-2.98 (m, 1H), 2.63-2.52 (m, 1H), 2.51 (s, 3H).
-
Lead Reference
-
- Synthesis and applications of S-(trifluoromethyl)-2,8-bis(trifluoromethoxy)dibenzothiophenium triflate (Umemoto reagent IV)
-
Other References
-
- Introduction of Fluorine and Fluorine-Containing Functional Groups

References
- Introduction of Fluorine and Fluorine-Containing Functional Groups
- Synthesis and applications of S-(trifluoromethyl)-2,8-bis(trifluoromethoxy)dibenzothiophenium triflate (Umemoto reagent IV)
[Featured Products] Electrophilic Trifluoromethylating Agent, Umemoto Reagent IV / Electrophilic Fluorinating Agent, NFBB
化学品安全说明书(SDS)
如需更多帮助,请联系我 们。
技术规格
CoA及其他文档
示例 CoA
目前没有该产品的 CoA 示例。
分析图谱
很抱歉,您搜索的分析图谱无法提供。






