Maximum quantity allowed is 999
请选择数量
CAS RN: 719-98-2 | 产品编码: T3143
N-(Trifluoromethylthio)phthalimide

| 产品编码 | T3143 |
| 纯度/分析方法 | >98.0%(GC) |
| 分子式/分子量 | C__9H__4F__3NO__2S = 247.19 |
| 外观与形状(20°C) | 固体 |
| 储存温度 | 室温 (15°C以下阴凉干燥处) |
| 储存在惰性气体下 | 存放于惰性气体之中 |
| 应避免的情况 | 空气 |
| 包装和容器 | 1G-Glass Bottle with Plastic Insert (查看图片) |
| CAS RN | 719-98-2 |
| Reaxys-RN | 1464619 |
| PubChem物质ID | 253662481 |
技术规格
| Appearance | White to Almost white powder to crystal |
| Purity(GC) | min. 98.0 % |
| Melting point | 111.0 to 115.0 °C |
物性(参考值)
| 熔点 | 113 °C |
GHS
| 象形图 |

|
| 信号词 | Warning |
| 危险性说明 | H315 : Causes skin irritation. H319 : Causes serious eye irritation. |
| 防范说明 | P264 : Wash skin thoroughly after handling. P280 : Wear protective gloves/ eye protection/ face protection. P302 + P352 : IF ON SKIN: Wash with plenty of water. P337 + P313 : If eye irritation persists: Get medical advice/ attention. P305 + P351 + P338 : IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. P362 + P364 : Take off contaminated clothing and wash it before reuse. P332 + P313 : If skin irritation occurs: Get medical advice/ attention. |
相关法规
运输信息
| HS编码* | 2930.90-900 |
应用
Catalytic Trifluoromethylthiolation Using N-(Trifluoromethylthio)phthalimide
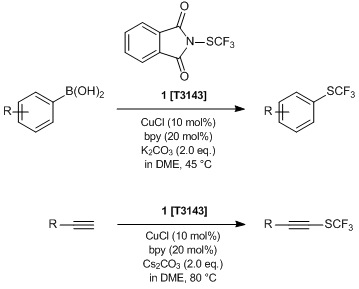
Typical Procedure (Reaction of 1 with boronic acids)3):
CuCl (4.0 mg, 0.04 mmol, 10 mol%), dry boronic acid (0.4 mmol, 1.0 eq.), dry K2CO3 (111 mg, 0.8 mmol, 2.0 eq.), 2,2’-bipyridine (12.5 mg, 0.08 mmol, 20 mol%) and N-(trifluoromethylthio)phthalimide (103.8 mg, 0.42 mmol, 1.05 eq.) are placed into an oven-dried Schlenk tube equipped with a stirring bar. The tube is sealed with a rubber stopper, evacuated and back-filled with dry argon (three times). Dry, freshly distilled and degassed DME is added by a syringe and the reaction mixture is stirred at 45 °C for 18 h. The reaction mixture is cooled to room temperature, diluted with diethyl ether (5 mL), and filtered through a short plug of silica, eluting with additional diethyl ether (50 mL). The filtrate is concentrated; pentane (10 mL) is added and concentrated again for removing residual DME. The resulting residue is purified by chromatography (pentane―diethyl ether) to provide the desired product.
CuCl (4.0 mg, 0.04 mmol, 10 mol%), dry boronic acid (0.4 mmol, 1.0 eq.), dry K2CO3 (111 mg, 0.8 mmol, 2.0 eq.), 2,2’-bipyridine (12.5 mg, 0.08 mmol, 20 mol%) and N-(trifluoromethylthio)phthalimide (103.8 mg, 0.42 mmol, 1.05 eq.) are placed into an oven-dried Schlenk tube equipped with a stirring bar. The tube is sealed with a rubber stopper, evacuated and back-filled with dry argon (three times). Dry, freshly distilled and degassed DME is added by a syringe and the reaction mixture is stirred at 45 °C for 18 h. The reaction mixture is cooled to room temperature, diluted with diethyl ether (5 mL), and filtered through a short plug of silica, eluting with additional diethyl ether (50 mL). The filtrate is concentrated; pentane (10 mL) is added and concentrated again for removing residual DME. The resulting residue is purified by chromatography (pentane―diethyl ether) to provide the desired product.
References
- 1)Trifluoromethylsulfenylation of masked carbonyl compounds
- 2)N-Trifluoromethylthiophthalimide: a stable electrophilic SCF3-reagent and its application in the catalytic asymmetric trifluoromethylsulfenylation
- 3)Direct catalytic trifluoromethylthiolation of boronic acids and alkynes employing electrophilic shelf-stable N-(trifluoromethylthio)phthalimide
参考文献
文章/手册
产品文档 (部分产品的分析图谱无法提供,敬请谅解。)






