Maximum quantity allowed is 999
请选择数量
CAS RN: 34946-82-2 | 产品编码: T1292
Copper(II) Trifluoromethanesulfonate

| 规格 | 单价 | 同一天 | 2-3个工作日 | 数量 |
|---|---|---|---|---|
| 5G |
S$85.50
|
10 | ≥100 |
|
| 25G |
S$261.00
|
11 | 34 |
|
技术规格
| Appearance | White to Gray to Dark blue powder to crystal |
| Purity(Chelometric Titration) | min. 98.0 % |
物性(参考值)
| 水溶性 | 可溶 |
GHS
| 象形图 |

|
| 信号词 | Danger |
| 危险性说明 | H314 : Causes severe skin burns and eye damage. |
| 防范说明 | P501 : Dispose of contents/ container to an approved waste disposal plant. P260 : Do not breathe dusts or mists. P264 : Wash skin thoroughly after handling. P280 : Wear protective gloves/ protective clothing/ eye protection/ face protection. P303 + P361 + P353 : IF ON SKIN (or hair): Take off immediately all contaminated clothing. Rinse skin with water/shower. P301 + P330 + P331 : IF SWALLOWED: Rinse mouth. Do NOT induce vomiting. P363 : Wash contaminated clothing before reuse. P304 + P340 + P310 : IF INHALED: Remove person to fresh air and keep comfortable for breathing. Immediately call a POISON CENTER/doctor. P305 + P351 + P338 + P310 : IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. Immediately call a POISON CENTER/doctor. P405 : Store locked up. |
相关法规
运输信息
| UN编号 | UN1759 |
| 类别 | 8 |
| 包装类别 | III |
| HS编码* | 2904.10-000 |
应用
Copper (II) Triflate Catalyzed Regioselective Markovnikov Hydration of Alkynes

Typical Procedure (R1 = 4-methoxybenznene, R2 = H): Cu(OTf)2 (72 mg, 20 mol%) and water (36 mg, 2 eq.) are added to a stirred solution of 1-ethynyl-4-methoxybenzene (132.2 mg, 1 mmol) in EtOAc (2 mL). The reaction mixture is stirred at 100 °C for 1 hr. After completion of the reaction, it is cooled to room temperature; water is added and extracted with EtOAc, dried over anhydrous Na2SO4 and concentrated. The residue is purified by column chromatography to give 4'-methoxyacetophenone (135 mg, 90%) as a white solid.
References
应用
Synthesis of Optically Active Oxazoline Derivatives via Asymmetric Desymmetrization of 1,3-Diols
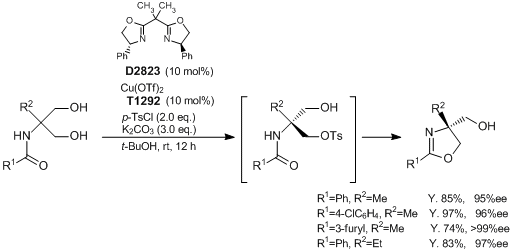
Typical Procedure:
Amino diols (0.5 mmol), K2CO3 (207.3 mg, 1.5 mmol), and p-toluenesulfonyl chloride (190.7 mg, 1.0 mmol) are added to a solution of Cu(OTf)2 (18.1 mg, 0.05 mmol) and (R,R)-2,2'-isopropylidenebis(4-phenyl-2-oxazoline) (16.7 mg, 0.05 mmol) in tert-butyl alcohol (2 mL). After stirring for 12 h at room temperature, water (10 mL) is added. The resulting mixture is extracted with AcOEt. After separation, the organic layer is washed with brine, and then dried over Na2SO4. Concentration and purification through flash column chromatography gives the product.
Amino diols (0.5 mmol), K2CO3 (207.3 mg, 1.5 mmol), and p-toluenesulfonyl chloride (190.7 mg, 1.0 mmol) are added to a solution of Cu(OTf)2 (18.1 mg, 0.05 mmol) and (R,R)-2,2'-isopropylidenebis(4-phenyl-2-oxazoline) (16.7 mg, 0.05 mmol) in tert-butyl alcohol (2 mL). After stirring for 12 h at room temperature, water (10 mL) is added. The resulting mixture is extracted with AcOEt. After separation, the organic layer is washed with brine, and then dried over Na2SO4. Concentration and purification through flash column chromatography gives the product.
References
应用
Efficient preparation method for alpha, omega-amino alcohols

Reference
参考文献
产品文档 (部分产品的分析图谱无法提供,敬请谅解。)
化学品安全说明书(SDS)
请选择语言。
如需更多帮助,请联系我 们。
技术规格
CoA及其他文档
请输入批号
批号输入有误。请输入中横线前的4-5个字母数字字符。
示例 CoA
可下载CoA示例。注:该示例不一定是最新批次的CoA。
目前没有该产品的 CoA 示例。
分析图谱
请输入批号
批号输入有误。请输入中横线前的4-5个字母数字字符。
很抱歉,您搜索的分析图谱无法提供。





