Maximum quantity allowed is 999
CAS RN: 361447-81-6 | 产品编码: P2822
1-Phenyl-1H-imidazol-3-ium Trifluoromethanesulfonate

| Appearance | White to Light yellow powder to crystal |
| Purity(HPLC) | min. 98.0 area% |
| Melting point | 126.0 to 131.0 °C |
| NMR | confirm to structure |
| 熔点 | 130 °C |
| 象形图 |

|
| 信号词 | Warning |
| 危险性说明 | H315 : Causes skin irritation. H319 : Causes serious eye irritation. |
| 防范说明 | P264 : Wash skin thoroughly after handling. P280 : Wear protective gloves/ eye protection/ face protection. P302 + P352 : IF ON SKIN: Wash with plenty of water. P337 + P313 : If eye irritation persists: Get medical advice/ attention. P305 + P351 + P338 : IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. P362 + P364 : Take off contaminated clothing and wash it before reuse. P332 + P313 : If skin irritation occurs: Get medical advice/ attention. |
| HS编码* | 2933.29-000 |

-
Used Chemicals
-
Procedure
-
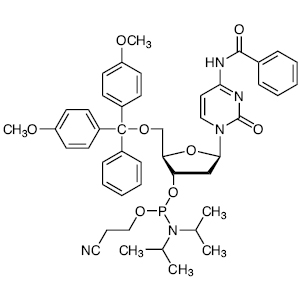
To an acetonitrile (2 mL) solution of 2-cyanoethyl N,N,N',N'-tetraisopropylphosphordiamidite (0.42 g, 1.4 mmol) and N-PhIMT (0.34 g, 1.2 mmol) were added a DMF solution of 1 (0.40 g, 1.2 mmol) under nitrogen atmosphere. The reaction mixture was stirred overnight at room temperature, then diluted with TBME and washed with H2O, DMF:H2O (1:1) solution, H2O and brine. The organic layer was concentrated under reduced pressure. The resulting crude product was purified by column chromatography (hexane:ethyl acetate:triethylamine = 1:2:0.03 on silica gel) to give 2 as a white powder (0.43 g, 66% yield).
-
Experimenter’s Comments
-
The reaction mixtures were monitored by LCMS.
-
Analytical Data
-
Compound 2
1H NMR (400 MHz, DMSO-d6); δ 11.36 (brs, 1H), 8.05-7.96 (m, 2H), 7.73-7.64 (m, 1H), 7.59-7.51 (m, 2H), 7.45-7.37 (m, 1H), 6.25-6.17 (m, 1H), 4.72-4.62 (m, 1H), 4.62-4.52 (m, 1H), 4.51-4.38 (m, 1H), 4.32-4.15 (m, 1H), 3.84-3.66 (m, 2H), 3.66-3.51 (m, 2H), 2.84-2.73 (m, 2H), 2.47-2.22 (m, 2H), 1.67-1.53 (m, 3H), 1.27-1.03 (m, 12H).
13C NMR (101 MHz, DMSO-d6); δ 165.5, 163.6, 150.4, 135.8, 133.7, 129.3 (3C), 128.9 (2C), 119.0, 109.9, 84.1, 82.8, 72.8, 64.9, 58.3 (2C), 42.7, 37.7, 24.3 (4C), 19.8, 11.9.
31P NMR (162 MHz, DMSO-d6); δ 147.89.
-
Lead Reference
-
- Acid/Azole Complexes as Highly Effective Promoters in the Synthesis of DNA and RNA Oligomers via the Phosphoramidite Method
References
- N-phenylimidazolium triflate as a highly effective promoter for the interribonucleotide-bond formation via the phosphoramidite method
- Acid/Azole Complexes as Highly Effective Promoters in the Synthesis of DNA and RNA Oligomers via the Phosphoramidite Method
- Utility of azolium triflates as promoters for the condensation of a nucleoside phosphoramidite and a nucleoside in the Agrawal's stereoselective synthesis of nucleoside phosphorothioates
- Nucleosidic Phosphoramidite Synthesis via Phosphitylation: Activator Selection and Process Development
- Benzimidazolium triflate (a review)
文章/手册
化学品安全说明书(SDS)
如需更多帮助,请联系我 们。
技术规格
CoA及其他文档
示例 CoA
目前没有该产品的 CoA 示例。
分析图谱
很抱歉,您搜索的分析图谱无法提供。