Maximum quantity allowed is 999
CAS RN: 4023-02-3 | 产品编码: A2055
1-Amidinopyrazole Hydrochloride

| Appearance | White to Yellow to Orange powder to crystal |
| Purity(HPLC) | min. 98.0 area% |
| Purity(Nonaqueous Titration) | min. 98.0 % |
| Melting point | 165.0 to 169.0 °C |
| 熔点 | 167 °C |
| 象形图 |

|
| 信号词 | Warning |
| 危险性说明 | H315 : Causes skin irritation. H319 : Causes serious eye irritation. |
| 防范说明 | P264 : Wash skin thoroughly after handling. P280 : Wear protective gloves/ eye protection/ face protection. P302 + P352 : IF ON SKIN: Wash with plenty of water. P337 + P313 : If eye irritation persists: Get medical advice/ attention. P305 + P351 + P338 : IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. P362 + P364 : Take off contaminated clothing and wash it before reuse. P332 + P313 : If skin irritation occurs: Get medical advice/ attention. |
| HS编码* | 2933.19-000 |

-
Used Chemicals
-
Procedure
-
A solution of 4-bromophenethylamine (200 mg, 1.00 mmol), DIEA (509 µL, 3.00 mmol) and praxadine (147 mg, 1.00 mmol) in DMF (1.7 mL) was stirred at ambient temperature for 2 days. The reaction mixture was concentrated and the residue was washed with dichloromethane/2-propanol (4:1 (vol/vol)). The guanidinylated compound 1 was obtained as a pale yellow solid (164 mg, 67% yield.) after filtration.
-
Experimenter’s Comments
-
The reaction mixture was monitored by LC/MS.
-
Analytical Data
-
Guanidinylated Compound 1
1H NMR (270 MHz, DMSO-d6); δ 7.57 (t, J = 6.3 Hz, 1H), 7.52 (d, J = 8.4 Hz, 2H), 7.25 (d, J = 8.4 Hz, 2H), 6.99 (brs, 3H), 3.35 (q, J = 6.3 Hz, 2H), 2.76 (t, J = 6.3 Hz, 2H).
-
Lead Reference
-
- Synthesis and pharmacological characterization of a new benzoxazole derivative as a potent 5-HT3 receptor agonist
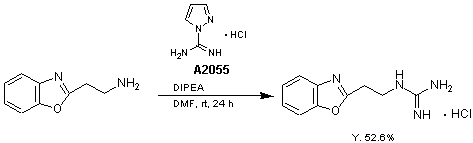
1-Amidinopyrazole hydrochloride (1 g, 6.8 mmol) is added to a solution of 2-(benzoxazol-2-yl)ethylamine (1.1 g, 6.7 mmol) and diisopropylethylamine (4.75 mL, 27 mmol) in DMF (10 mL). The reaction mixture is stirred at room temperature under N2 for 24 h. Subsequently, diethylether (75 mL) is added, the upper layer is decanted and the remaining oil solidified by treatment with CH2Cl2. The solid is collected, washed with diethylether, CH2Cl2, and CHCl3 : i-PrOH (4 : 1) and dried to yield the product (0.86 g, Y. 52.6%).
References
- P. L. Lopez-Tudanca, L. Labeaga, A. Innerarity, L. Alonso-Cires, I. Tapia, R. Mosquera, A. Orjales, Bioorg. Med. Chem. 2003, 11, 2709.

- Y. Zhang, A. J. Kennan, Org. Lett. 2001, 3, 2341.

- A. Porcheddu, G. Giacomelli, A. Chighine, S. Masala, Org. Lett. 2004, 6, 4925.

化学品安全说明书(SDS)
如需更多帮助,请联系我 们。
技术规格
CoA及其他文档
示例 CoA
目前没有该产品的 CoA 示例。
分析图谱
很抱歉,您搜索的分析图谱无法提供。






