Haruhiko Taguchi
Tokyo Chemical Industry, Co. Ltd.
Maximum quantity allowed is 999
Please select the quantity
Short Topic: More ways to use reagents |
Synthesis of Multi-substituted Olefins via SCOOPY Reaction
No.159(October 2013)
In this column, we focus on another usage of reagents from the viewpoint of the reagent company. In this issue, we introduce a certain name reaction with its research progress.
Name reactions are very important synthetic methods in organic synthesis because their discoveries have developed the progress of organic chemistry. I think young chemists study hard to develop novel and innovative reactions and to be used as a name reaction in the future. Similarly, named reagents are available from reagent companies. For chemists, it would be great honor to have their developed compound as a named reagent. In this way, name reactions and named reagents motivate them to study hard day and night.
On the other hand, examples such as metathesis reaction and malonic ester synthesis, the names of which are derived from the concept of each reaction, are also categorized as name reactions though they aren’t people’s names. I think “name reaction” is a useful term since various types of organic reactions can be expressed by a suitable single phrase.
Now, we are easily able to get the information about name reactions through the internet and a book titled “STRATEGIC APPLICATIONS of NAMED REACTIONS in ORGANIC SYNTHESIS”, which has been published and 250 named reactions are explained in detail. This book is good for study of organic reactions. When I read it, I remembered a certain name reaction.
The name of this reaction is the SCOOPY reaction. I’m sure if you are fully aware of organic chemistry, you will well know that reaction. I searched for the SCOOPY reaction using the internet but I could collect only little related information. It seems that a chemically similar reaction named the Wittig-Schlosser reaction attracts chemists more compared with the SCOOPY reaction. Due to that reason, the SCOOPY reaction would be not widely known. However, the SCOOPY reaction has an advantage in the synthesis of trisubstituted olefins with high stereoselectively.
By the way SCOOPY, who are you? SCOOPY says “I’m not a man, not a people’s name. I’m an abbreviated word!”. “α-Substitution plus Carbonyl Olefination via β-Oxido Phosphorus Ylides”, which is abbreviated to S.C.O.O.P.Y.1) This word is named by Prof. M. Schlosser, a developer of the Wittig-Schlosser reaction. We can understand the concept of S.C.O.O.P.Y. from the above sentence as following, “to achieve both α-substitution and carbonylolefination utilizing the chemical property of β-oxido phosphorus ylides”. Well, what chemical properties do β-oxido phosphorus ylides have?
β-Oxido phosphorus ylides are generally formed by the further reaction of betains as intermediates of the Wittig reaction with one equivalent of phenyl lithium. Initially, the erythro form of β-oxido phosphorus ylide is kinetically formed as the main intermediate and then it is rapidly transformed to the more stable threo form, because the rate of isomerization between them is sufficiently fast under low temperature.

M. Schlosser and his coworker have discovered the above-mentioned chemical properties of β-oxido phosphorus ylides and applied them for novel organic synthesis. They have reported disubstituted olefins are stereospecifically given by the treatment of the threo form of β-oxido phosphorus ylide with tert-butanol. This synthetic manner is later named as the Wittig-Schlosser reaction.
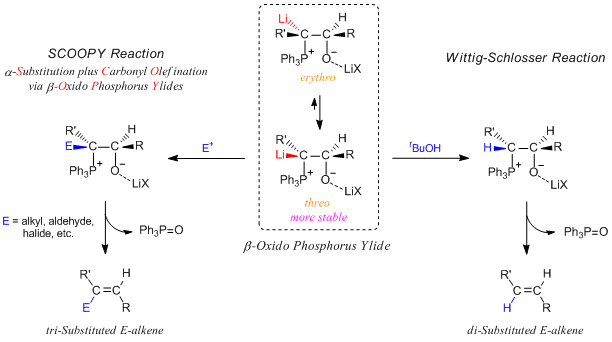
Furthermore, they published the report titled “Carbonyl Olefination with α-Substitution” in the first issue of “SYNTHESIS” in 1969.2) The phrase in the title “with α-Substitution” can be expressed in “with α-Substitution of β-oxido phosphorus ylides”. In this report, they showed that β-oxido phosphorus ylides can be α-substituted by methyl iodide to afford trisubstituted olefins stereoselectively.
This reaction is later called the SCOOPY reaction, but at that time no one had called this reaction such a name. The concept of “Carbonyl Olefination with α-Substitution” attracted many chemists and some of whom had studied on the reactivities of β-oxido phosphorus ylides with various electrophiles.
Then, in 1971, M. Schlosser and his coworker reported a paper including the phrase “S.C.O.O.P.Y” in its title in the journal of “SYNTHESIS”.1) The opening sentence of it is magnificently written and we can feel their interest and inspiration at that time even after over 40 years have passed.
The SCOOPY reaction has been often used to produce trisubsituted olefins in fine organic chemistry, especially, synthetic chemistry of natural compounds. This reaction is very excellent but it is very difficult to control the stereochemistry with 100% selectively and in some cases, given that the reaction products include almost same equivalent of E : Z mixture.
It seems that the SCOOPY reaction is a minor name reaction in spite of that the chemical property of β-oxido phosphorus ylides is very attractive due to the similar reaction named the Wittig-Schlosser reaction is very famous name reaction. However, the SCOOPY reaction gives one effective tool to synthesize trisubsituted olefins with high stereoselectivity. The process of the S.C.O.O.P.Y, which utilizes the chemical property of phosphorus to develop novel organophosphorus chemistry is recommended to be used with these two name reactions, the SCOOPY reaction and the Wittig-Schlosser reaction, for your organic synthesis.
References
The prices are subject to change without notice. Please confirm the newest price by our online catalog before placing an order.
In addition, sales products changes with areas. Please understand that a product is not available when the product details page is not displayed.
In addition, sales products changes with areas. Please understand that a product is not available when the product details page is not displayed.

