Maintenance Notice (3:15 - 7:30 AM February 22, 2025 UTC): This website is scheduled to be unavailable due to maintenance. We appreciate your patience and understanding.
Notice of Discontinuing the Use of Password-Protected Compressed Files | Product Document Searching Made Easy by 2D Code! | TCI Life Science News February 2025 | [Product Highlights] Chemiluminescent Reagents for... | Various analytical charts can be searched on each product detail page and Product Document Search (The kinds of analytical charts differ by product)
Maximum quantity allowed is 999
Please select the quantity
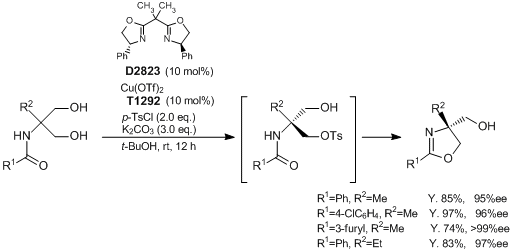
Synthesis of Optically Active Oxazoline Derivatives via Asymmetric Desymmetrization of 1,3-Diols
Onomura et al. have reported the synthesis of optically active oxazolines through a copper-catalyzed asymmetric desymmetrization of 1,3-diols. According to their results, 2-(N-acylamino)-1,3-propanediols react with p-toluenesulfonyl chloride in the presence of Cu(OTf)2 and (R,R)-2,2'-isopropylidenebis(4-phenyl-2-oxazoline) as a catalyst to give the desired optically active oxazoline derivatives having a quaternary stereocenter in good to excellent yields with high enantioselectivities. The advantages of this method are a simple and easy operation and mild reaction conditions. Since this reaction system tolerates a diverse range of substrates, it is expected to be applied to the synthesis of various biologically active agents.