Maximum quantity allowed is 999
Please select the quantity
CAS RN: 4136-95-2 | Product Number: T1413
2,4,6-Trichlorobenzoyl Chloride

Purity: >98.0%(GC)(T)
Synonyms:
Product Documents:
| Size | Unit Price | Same Day | 2-3 Business Days | Other Lead Time | Quantity |
Shipping Information

|
|---|---|---|---|---|---|---|
| 5G |
S$133.50
|
6 | 0 | Contact Us |
|
|
| 25G |
S$462.00
|
30 | 6 | Contact Us |
|
* Please contact our distributors or
TCI
to order TCI products.
* The storage conditions are subject to change without notice.
| Product Number | T1413 |
| Purity / Analysis Method | >98.0%(GC)(T) |
| Molecular Formula / Molecular Weight | C__7H__2Cl__4O = 243.89 |
| Physical State (20 deg.C) | Liquid |
| Storage Temperature | Room Temperature (Recommended in a cool and dark place, <15°C) |
| Store Under Inert Gas | Store under inert gas |
| Condition to Avoid | Moisture Sensitive |
| CAS RN | 4136-95-2 |
| Reaxys Registry Number | 2050280 |
| PubChem Substance ID | 87577179 |
| MDL Number | MFCD00075323 |
Specifications
| Appearance | Colorless to Light yellow clear liquid |
| Purity(GC) | min. 98.0 % |
| Purity(Argentometric Titration) | min. 98.0 % |
Properties (reference)
| Boiling Point | 275 °C |
| Specific Gravity (20/20) | 1.56 |
| Refractive Index | 1.58 |
| Solubility (soluble in) | Toluene |
GHS
| Pictogram |

|
| Signal Word | Danger |
| Hazard Statements | H314 : Causes severe skin burns and eye damage. H290 : May be corrosive to metals. |
| Precautionary Statements | P501 : Dispose of contents/ container to an approved waste disposal plant. P234 : Keep only in original container. P264 : Wash skin thoroughly after handling. P280 : Wear protective gloves/ protective clothing/ eye protection/ face protection. P390 : Absorb spillage to prevent material damage. P303 + P361 + P353 : IF ON SKIN (or hair): Take off immediately all contaminated clothing. Rinse skin with water/shower. P301 + P330 + P331 : IF SWALLOWED: Rinse mouth. Do NOT induce vomiting. P363 : Wash contaminated clothing before reuse. P304 + P340 + P310 : IF INHALED: Remove person to fresh air and keep comfortable for breathing. Immediately call a POISON CENTER/doctor. P305 + P351 + P338 + P310 : IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. Immediately call a POISON CENTER/doctor. P406 : Store in corrosive resistant container with a resistant inner liner. P405 : Store locked up. |
Related Laws:
Transport Information:
| UN Number | UN3265 |
| Class | 8 |
| Packing Group | II |
| H.S.code* | 2916.39-000 |
Application
Yamaguchi Esterification and Macrolactonization
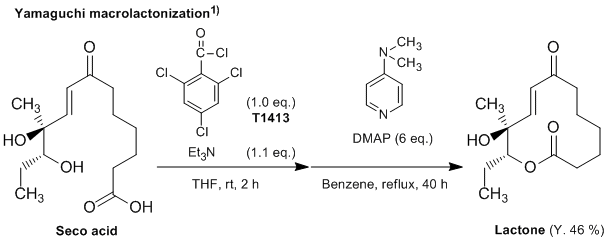
2,4,6-Trichlorobenzoyl chloride is often used for the Yamaguchi esterification and macrolactonization. The Yamaguchi esterification is the rapid and mild esterification method in the presence of 4-dimethylaminopyridine (DMAP) using a mixed anhydride, which is prepared from 2,4,6-trichlorobenzoyl chloride and the carboxylic acid. 1–3) The features of this esterification are as follows: 1) the reaction is conducted under high-dilution conditions to minimize intermolecular coupling; 2) the mixed anhydride is dissolved and slowly added to a refluxing solution of DMAP in aromatic hydrocarbons (e.g. benzene, toluene); 3) usually several equivalents of DMAP, as a catalyst for acylation, are used. It is especially useful in the synthesis of macrolactones with intramolecular esterification, called the Yamaguchi macrolactonization. 1,4)
Typical procedure1): A mixture of the seco acid (272 mg) and triethylamine (153 µL) in THF (10 mL) is stirred for 10 min at room temperature, and then 2,4,6-trichlorobenzoyl chloride (160 µL) is added. After stirring for 2 h at room temperature, the resulting precipitate is filtered and washed with a small amount of THF. The filtrate is diluted with benzene (500 mL) and slowly added to a refluxing solution of DMAP (732 mg) in benzene (100 mL) over a period of 40 h. The reaction mixture is washed successively with a saturated aqueous citric acid solution, water, an aqueous sodium hydrogen carbonate, and water, and dried over anhydrous magnesium sulfate. Concentration of the filtrate under reduced pressure affords a crude product. The product is separated by preparative TLC (eluent: diethyl ether / benzene = 2 / 1) on silica gel to give 116 mg of the lactone (46 % yield).
References
- 1)Seminal Publications
- 2)Reviews
- 3)Theoretical Studies
- 4)Stereoselective Synthesis of Macrolides
- a)M. Hikota, H. Tone, K. Horita O. Yonemitsu, Tetrahedron 1990, 46, 4613.

- b)A. K. Ghosh, Y. Wang, J. T. Kim, J. Org. Chem. 2001,66, 8973.

- c)J. P. Marino, M. S. McClure, D. P. Holub, J. V. Comasseto, F. C. Tucci, J. Am. Chem. Soc., 2002, 124, 1664.

- d)T. Hu, N. Takenaka, J. S. Panek, J. Am. Chem. Soc., 2002, 124, 12806.

- a)M. Hikota, H. Tone, K. Horita O. Yonemitsu, Tetrahedron 1990, 46, 4613.
PubMed Literature
Articles/Brochures
TCIMAIL
Product Documents (Note: Some products will not have analytical charts available.)
Safety Data Sheet (SDS)
Please select Language.
The requested SDS is not available.
Please Contact Us for more information.
Specifications
C of A & Other Certificates
Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
Sample C of A
This is a sample C of A and may not represent a recently manufactured lot of the product.
A sample C of A for this product is not available at this time.
Analytical Charts
Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
The requested analytical chart is not available. Sorry for the inconvenience.





