Maximum quantity allowed is 999
CAS RN: 90965-06-3 | Product Number: D3546
Dimethyl (1-Diazo-2-oxopropyl)phosphonate

Purity: >97.0%(HPLC)
- (1-Diazo-2-oxopropyl)phosphonic Acid Dimethyl Ester
- Dimethyl 1-Diazoacetonylphosphonate
- 1-Diazoacetonylphosphonic Acid Dimethyl Ester
- Ohira-Bestmann Reagent
| Size | Unit Price | Same Day | 2-3 Business Days | Other Lead Time | Quantity |
Shipping Information

|
|---|---|---|---|---|---|---|
| 1G |
S$223.50
|
10 | 29 | Contact Us |
|
|
| 5G |
S$790.50
|
≥40 | ≥40 | Contact Us |
|
* Please contact our distributors or
TCI
to order TCI products.
* The storage conditions are subject to change without notice.
| Product Number | D3546 |
| Purity / Analysis Method | >97.0%(HPLC) |
| Molecular Formula / Molecular Weight | C__5H__9N__2O__4P = 192.11 |
| Physical State (20 deg.C) | Liquid |
| Storage Temperature | Refrigerated (0-10°C) |
| Store Under Inert Gas | Store under inert gas |
| Condition to Avoid | Air Sensitive,Heat Sensitive |
| CAS RN | 90965-06-3 |
| Reaxys Registry Number | 4247670 |
| PubChem Substance ID | 87559944 |
| Merck Index (14) | 1177 |
| MDL Number | MFCD07368360 |
| Appearance | Light yellow to Brown clear liquid |
| Purity(HPLC) | min. 97.0 area% |
| Specific Gravity (20/20) | 1.28 |
| Refractive Index | 1.48 |
| H.S.code* | 2931.49-900 |

-
Used Chemicals
-
Procedure
-
To a methanol (10 mL) solution of undecanal (0.20 g, 1.2 mmol) was added potassium carbonate (0.32 g,2.4 mmol) and Ohira-Bestmann reagent (0.27 g, 1.4 mmol) at room temperature. The reaction mixture was stirred overnight at room temperature, then diluted with diethyl ether and washed with saturated aqueous sodium bicarbonate, and dried by sodium sulfate. The organic layer was concentrated under reduced pressure. The resulting crude product was purified by column chromatography (hexane:toluene = 3:1 on silica gel) to give 1 as a colorless liquid (0.11 g, 56% yield).
-
Experimenter’s Comments
-
The reaction mixtures were monitored by 1H NMR (CDCl3).
-
Analytical Data(1-Dodecyne (1))
-
1H NMR (400 MHz, CDCl3); δ 2.18 (t, 2H, J = 7.1 Hz), 1.94 (s, 1H), 1.57-1.46 (m, 2H), 1.44-1.33 (m, 2H), 1.33-1.19 (m, 12H), 0.88 (t, 3H, J = 6.4 Hz).
13C NMR (101 MHz, CDCl3); δ 85.0, 68.2, 32.0, 29.7, 29.7, 29.5, 29.3, 28.9, 28.6, 22.8, 18.5, 14.3.
-
Lead Reference
-
- Further Improvements of the Synthesis of Alkynes from Aldehydes
-
Other References
-
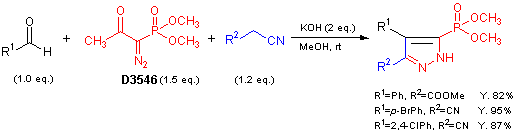
- Methanolysis of Dimethyl (1-Diazo-2-oxopropyl) Phosphonate: Generation of Dimethyl (Diazomethyl) Phosphonate and Reaction with Carbonyl Compounds
References

References
- S. Ohira, Synth. Commun. 1989, 19, 561.

- S. Muller, B. Liepold, G. J. Roth, H. J. Bestmann, Synlett 1996, 521.

- H. D. Dickson, S. C. Smith, K. W. Hinkle, Tetrahedron Lett. 2004, 45, 5597.

- E. Quesada, R. J. K. Taylor, Tetrahedron Lett. 2005, 46, 6473.

Articles/Brochures
[TCI Practical Example] Construction of the Terminal Alkyne Using Ohira-Bestmann Reagent
Safety Data Sheet (SDS)
The requested SDS is not available.
Please Contact Us for more information.
Specifications
C of A & Other Certificates
Sample C of A
A sample C of A for this product is not available at this time.
Analytical Charts
The requested analytical chart is not available. Sorry for the inconvenience.






