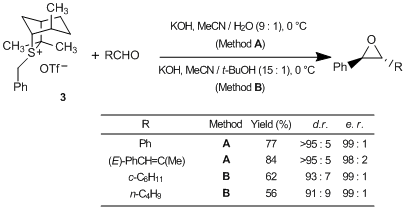
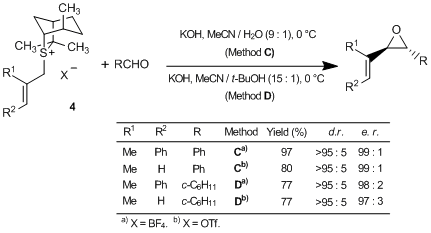
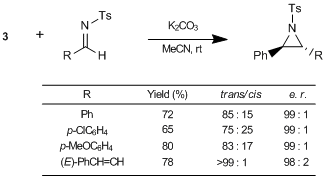
Aggarwal et al. have reported an asymmetric synthesis using the sulfur ylides generated in situ by treating the sulfonium salts 3 and 4 prepared from (1R,4R,5R)-4,7,7-trimethyl-6-thiabicyclo[3.2.1]octane (1), with bases. According to their results, the reactions of 3 with aldehydes and imines afford the corresponding chiral oxiranes and aziridines, respectively, in high yields with diastereo- and enantioselectivities.1) On the other hand, the reactions of 4 with aldehydes also give excellent yields of the corresponding α,β-unsaturated oxiranes with perfect stereoselectivities.1) Moreover, this methodology was also successfully applied to the total synthesis of alkaloids, such as quinine.1)
Thus, 1 and 2 are useful chiral auxiliaries applicable to the synthesis of various oxiranes and aziridines via sulfur ylides.
Maximum quantity allowed is 999
Please select the quantity
Useful Chiral Auxiliaries for the Synthesis of Chiral Oxiranes
No.149(December 2012)


References
- 1)Practical and highly selective sulfur ylide mediated asymmetric epoxidations and aziridinations using an inexpensive, readily available chiral sulfide
The prices are subject to change without notice. Please confirm the newest price by our online catalog before placing an order.
In addition, sales products changes with areas. Please understand that a product is not available when the product details page is not displayed.
In addition, sales products changes with areas. Please understand that a product is not available when the product details page is not displayed.