Maximum quantity allowed is 999
Please select the quantity
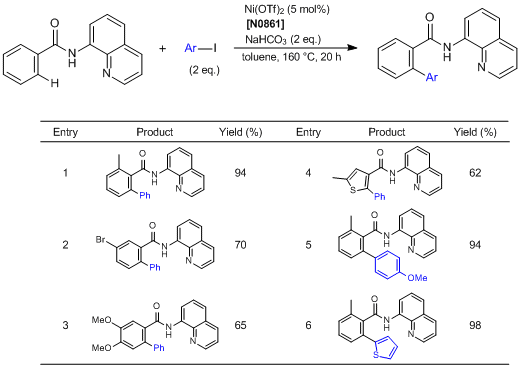
Direct Arylation of C—H Bonds in Aromatic Amides Containing an 8-Aminoquinoline Moiety
Chatani et al. have reported the direct arylation of C—H bonds in aromatic amides containing an 8-aminoquinoline moiety as a directing group. According to their results, the aromatic amides are regioselectively arylated via the cleavage of the ortho C—H bonds by the reaction with aryl iodides using nickel(II) trifluoromethanesulfonate as a catalyst. Various functional group-substituted aromatic amides are tolerated in the reaction. In the reaction using meta-substituted aromatic amides, the arylation proceeds in a highly selective manner at the less hindered C—H bonds, irrespective of the electronic nature of the substituents.