Maximum quantity allowed is 999
CAS RN: 12148-71-9 | Product Number: C2662
(1,5-Cyclooctadiene)(methoxy)iridium(I) Dimer

Purity:
- Bis(1,5-cyclooctadiene)di-μ-methoxydiiridium(I)
| Size | Unit Price | Same Day | 2-3 Business Days |
|---|---|---|---|
| 200MG |
$72.00
|
≥40 | 34 |
| 1G |
$246.00
|
≥100 | ≥40 |
* Please contact our distributors or
TCI
to order our products. The above prices do not include freight cost, customs, and other charges to the destination.
* The storage conditions are subject to change without notice.
| Product Number | C2662 |
| Molecular Formula / Molecular Weight | C__1__8H__3__0Ir__2O__2 = 662.87 |
| Physical State (20 deg.C) | Solid |
| Storage Temperature | Frozen (<0°C) |
| Store Under Inert Gas | Store under inert gas |
| Condition to Avoid | Light Sensitive,Air Sensitive,Moisture Sensitive,Heat Sensitive |
| Packaging and Container | 1G-Glass Bottle with Plastic Insert (View image), 200MG-Glass Bottle with Plastic Insert (View image) |
| CAS RN | 12148-71-9 |
| Reaxys Registry Number | 14520157 |
| PubChem Substance ID | 160871404 |
| MDL Number | MFCD08459360 |
| Appearance | Light yellow to Amber to Dark green powder to crystal |
| Elemental analysis(Carbon) | 31.50 to 34.00 % |
| Melting Point | 179 °C(dec.) |
| H.S.code* | 2843.90-000 |
-
Used Chemicals
-
Procedure
-
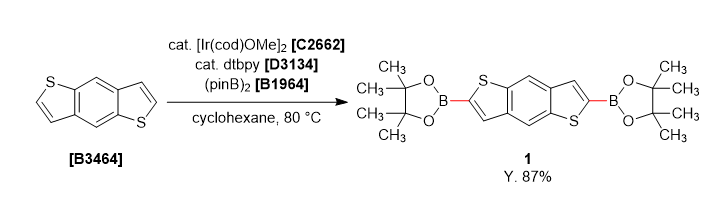
A solution of dtbpy (27 mg, 0.05 mmol), bis(pinacolato)diboron (1.0 g, 4.0 mmol) and [Ir(cod)OMe]2 (33 mg, 0.025 mmol) in cyclohexane (40 mL) was stirred under nitrogen at room temperature for 10 min. Benzo[1,2-b:4,5-b']dithiophene (380 mg, 2.0 mmol) was added the mixture and stirred 80 ˚C for 18 hours. The reaction mixture was quenched with water and separated both layers, extracted with dichloromethane. The organic phase was dried over anhydrous sodium sulfate and filtered. The solvent was removed under reduced pressure and the crude was washed with methanol (20 mL) to give 1 as a white solid (0.771 g, 87% yield).
-
Experimenter’s Comments
-
The reaction mixture was monitored by NMR.
Cyclohexane was bubbled with nitrogen before use.
-
Analytical Data
-
Compound 1
1H NMR (270 MHz, CDCl3); δ 8.36 (s, 2H), 7.90 (s, 2H), 1.39 (s, 24H).
-
Lead Reference
-
- Synthesis and Transistor Application of Bis[1]benzothieno[6,7‑d:6′,7′‑d′]benzo[1,2‑b:4,5‑b′]dithiophenes

Reference
- Room temperature borylation of arenes and heteroarenes using stoichiometric amounts of pinacolborane catalyzed by iridium complexes in an inert solvent

An alcohol or ketone substrate is dissolved in THF and treated with a freshly prepared solution of [Ir(cod)OMe]2 (0.05 mol%) in THF and then with neat Et2SiH2 (1.2 eq.). The resulting solution is stirred at room temperature (23 °C) until complete conversion of the alcohol or ketone. At the completion of the reaction, the corresponding diethyl(hydrido)silyl ether is observed. Then the reaction mixture is placed under high vacuum for 1 h. The concentrated diethyl(hydrido)silyl ether is sequentially treated with freshly prepared solutions of norbornene (1.2 eq.) in THF and [Ir(cod)OMe]2 (0.5 mol%) in THF, and then with a slurry of Me4phen (1.25 mol%) in THF. The resulting solution is stirred at room temperature for 1 h and then heated it at 80-120 °C until complete conversion to the corresponding oxasilolane is observed. Then the crude reaction mixture containing the oxasilolane is sequentially treated with MeOH, KHCO3 (2.5 eq.) and H2O2 (30% solution in H2O, 10 eq.), and the resulting mixture is stirred overnight at 50 °C. The reaction is carefully quenched with aq. NaHSO3, and the resulting mixture is extracted with EtOAc. The combined organic layer is sequentially washed with 1 M HCl and sat. NaHCO3, and then dried with MgSO4. The resulting organic layer is filtered through Celite and concentrated to provide the crude diol, which is either purified directly or after conversion to the corresponding acetate derivative through treatment with Ac2O and Et3N.
References
Articles/Brochures
[Research Articles] Catalytic Functionalization of Un-activated Primary C-H Bonds
Safety Data Sheet (SDS)
The requested SDS is not available.
Please Contact Us for more information.
Specifications
C of A & Other Certificates
Sample C of A
A sample C of A for this product is not available at this time.
Analytical Charts
The requested analytical chart is not available. Sorry for the inconvenience.



