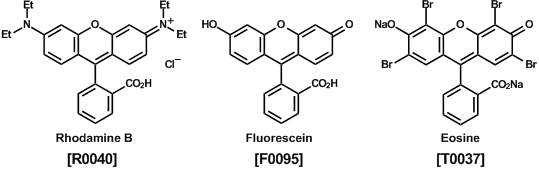
Both cationic and anionic xanthene dyes are known to be efficient fluorescent dyes. Functional groups on the xanthene moiety control their fluorescent colors. For instance, there are the cationic dyes of rhodamine B with a dialkylamino group as a red fluorescent dye, fluorescein as a green fluorescent dye, and the anionic dye of eosin (brominated fluorescein) as a red fluorescent dye. Applications of xanthene dyes involve optical materials and organic dyes for medical diagnosis research. Several characteristic features of xanthene dyes are large absorption and luminescence, excellent light resistance, low toxicity in-vivo, and relatively high solubility in water.
Since xanthene dyes have a sensitizing effect, their applications for dye sensitized solar cells (DSSC) have been reported.1) We can expect that the xanthene dyes fabricate DSSC devices at low cost, because they are metal-free, although the power conversion efficiency of xanthene dyes are lower than those of ruthenium-based dyes. Further research has been in progress with a combination of several xanthene dyes to increase power conversion efficiency.2)
There is an application of xanthene derivatives for a laser dye. A dye laser requires coumarin and rhodamine dyes as an organic medium. They all oscillate in the visible area. Among these xanthene dyes, the rhodamine 6G is mainly used for a laser dye.3)
References
- 1) H. Tsubomura, M. Matsumura, Y. Nomura, T. Amamiya, Nature 1976, 261, 402.

- 2) S. Rani, P. K. Shishodia, R. M. Mehra, J. Renew. Sust. Energ. 2010, 2, 043103.

- 3) J. Loerke, F. Marlow, Adv. Mater. 2002, 14, 1745.













