Maintenance Notice (3:30 - 10:00 AM December 21, 2024 UTC): This website is scheduled to be unavailable due to maintenance. We appreciate your patience and understanding.
Published TCIMAIL newest issue No.197 | Notice of Discontinuing the Use of Password-Protected Compressed Files | Product Document Searching Made Easy by 2D Code! | TCI Life Science News December 2024 | [TCIPracticalExample] Reduction of Amide Group Using... | Various analytical charts can be searched on each product detail page and Product Document Search (The kinds of analytical charts differ by product)
Maximum quantity allowed is 999
Please select the quantity
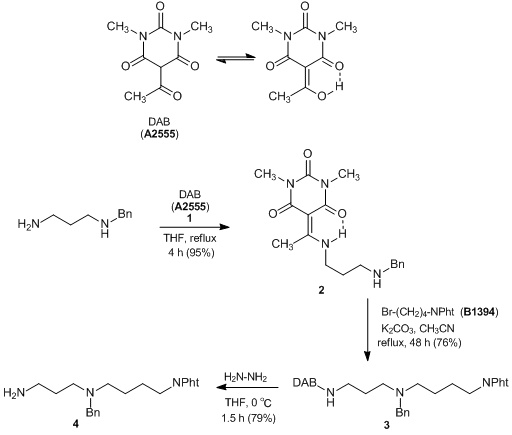
Selective Protection and Deprotection of Primary Amino Group Using 1,3-Dimethyl-5-acetylbarbituric Acid
1,3-Dimethyl-5-acetylbarbituric acid (DAB) (1) can selectively react with primary amines which have primary and secondary amino groups in molecules and affords desired enamine derivatives. Thus, 1 is used for the selective protection of primary amino groups. Deprotection of them is performed by the treatment with hydrazine giving the primary amino groups under mild conditions. As one example of protection and deprotection of primary amino groups using 1 for the preparation of the spermidine derivative 4, selective DAB protection of the primary amino group of (3-aminopropyl)benzylamine is demonstrated to afford a N-DAB adduct 2. This is then subjected to N-alkylation with N-(4-bromobutyl)phthalimide and subsequent selective DAB deprotection with aqueous hydrazine to obtain the spermidine derivative 4. This synthetic manner would be effective in selective protection / deprotection of the primary amino group of aminolinkers.