Maximum quantity allowed is 999
Please select the quantity
Catalytic Trifluoromethylthiolation Using N-(Trifluoromethylthio)phthalimide
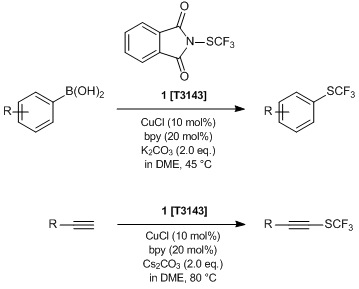
Rueping et al. have reported the catalytic trifluoromethylthiolation using N-(trifluoromethylthio)phthalimide (1). According to their results, 1 acts as an electrophilic source of CF3S+, and readily reacts with boronic acids and terminal alkynes under copper catalysis to afford the corresponding CF3S group containing compounds. 1 is a useful trifluoromethylthiolating agent, because it is shelf-stable and easy-to-handle, and the reaction proceeds under mild conditions. Now, the studies of the biological activities of CF3S group substituted compounds are attracting interest. So, 1 is expected to be applied for the synthesis of bioactive compounds as well as pharmaceutical and agrochemical research.
References
- Direct catalytic trifluoromethylthiolation of boronic acids and alkynes employing electrophilic shelf-stable N-(trifluoromethylthio)phthalimide