Maintenance Notice (10:30 PM January 4 - 8:00 AM January 5, 2025 UTC): This website is scheduled to be unavailable due to maintenance. We appreciate your patience and understanding.
Published TCIMAIL newest issue No.197 | Notice of Discontinuing the Use of Password-Protected Compressed Files | Various analytical charts can be searched on each product detail page and Product Document Search (The kinds of analytical charts differ by product)
Maximum quantity allowed is 999
Please select the quantity
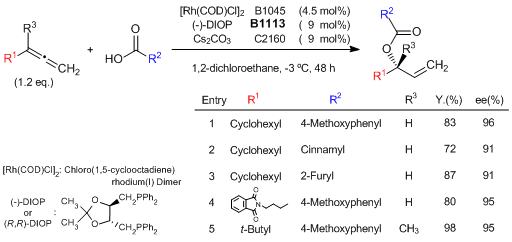
Rhodium(I)/(-)-DIOP Catalyzed Regio- and Enantioselective Addition of Carboxylic Acids to Allenes
Breit et al. have reported the first intermolecular regio- and enantioselective addition of carboxylic acids to terminal allenes to form branched allylic esters, employing a Rh(I)/(-)-DIOP catalyst system. Even 1,1-disubstituted allenes could be employed, leading to the formation of a quaternary chiral center. They have proposed the reaction proceeds through the Rh-π-allyl complex from the results of labeling experiments using deuterated benzoic acid.