Maximum quantity allowed is 999
Please select the quantity
CAS RN: 910110-45-1 | Product Number: H1407
(2S)-N-[(1S)-1-(Hydroxydiphenylmethyl)-3-methylbutyl]-2-pyrrolidinecarboxamide
![(2S)-N-[(1S)-1-(Hydroxydiphenylmethyl)-3-methylbutyl]-2-pyrrolidinecarboxamide No-Image](/medias/H1407.jpg?context=bWFzdGVyfHJvb3R8NTg5NTB8aW1hZ2UvanBlZ3xhRFJsTDJoaFlpODRPVEl5TURNMk1EUXpPREEyTDBneE5EQTNMbXB3Wnd8MTUxMjNjMjM3ZTU4ZTVjNzdiZmE1MjE4OGI4YzkyNjgwMmU1NGVkZWI4NThjNmFmZWNmZWExNmZjMzE2NGQ2Yg)
Purity: >98.0%(T)(HPLC)
Synonyms:
- (S)-N-[(S)-1-Hydroxy-4-methyl-1,1-diphenylpentan-2-yl]pyrrolidine-2-carboxamide
- N-[(1S)-1-(Hydroxydiphenylmethyl)-3-methylbutyl]-L-prolinamide
- Singh's Catalyst
Product Documents:
| Size | Unit Price | Same Day | 2-3 Business Days |
|---|---|---|---|
| 200MG |
$99.00
|
6 | 1 |
| 1G |
$312.00
|
1 | Contact Us |
* Please contact our distributors or
TCI
to order our products. The above prices do not include freight cost, customs, and other charges to the destination.
* The storage conditions are subject to change without notice.
| Product Number | H1407 |
| Purity / Analysis Method | >98.0%(T)(HPLC) |
| Molecular Formula / Molecular Weight | C__2__3H__3__0N__2O__2 = 366.51 |
| Physical State (20 deg.C) | Solid |
| Storage Temperature | Room Temperature (Recommended in a cool and dark place, <15°C) |
| CAS RN | 910110-45-1 |
| Reaxys Registry Number | 10473304 |
| PubChem Substance ID | 253661520 |
| MDL Number | MFCD22200506 |
Specifications
| Appearance | White to Almost white powder to crystal |
| Purity(HPLC) | min. 98.0 area% |
| Purity(Nonaqueous Titration) | min. 98.0 % |
| Melting point | 191.0 to 195.0 °C |
| Specific rotation [a]20/D | -46.0 to -49.0 deg(C=1, CHCl3) |
| NMR | confirm to structure |
Properties (reference)
| Melting Point | 193 °C |
| Specific Rotation | -47° (C=1,CHCl3) |
| Solubility (soluble in) | Methanol |
GHS
Related Laws:
Transport Information:
| H.S.code* | 2933.99-000 |
Application
An Efficient Chiral Organocatalyst for the Cross-aldol Reaction of Aliphatic Ketones with Aldehydes
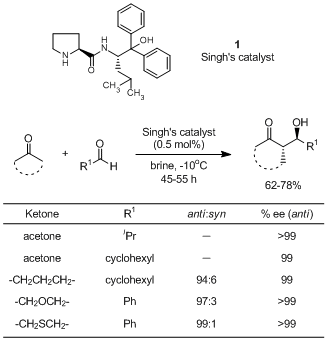
An aldehyde (0.5 mmol) is added to a mixture of ketone (2 mmol) and Singh's catalyst (0.5 mol %) in brine (0.5 mL) at -5 °C. The reaction mixture is stirred and the progress of the reaction is monitored by TLC. After reaction is over, the reaction mixture is diluted with EtOAc (10 mL). The organic layer is separated and dried over anhydrous Na2SO4. It is purified over silica gel by column chromatography to give the aldol product.
References
- Highly Efficient Small Organic Molecules for Enantioselective Direct Aldol Reaction in Organic and Aqueous Media
- Highly Enantioselective Organocatalytic Direct Aldol Reaction in an Aqueous Medium
- Highly Enantioselective Direct Aldol Reaction Catalyzed by Organic Molecules
- Enantioselective Aldol Reactions of Aliphatic Aldehydes with Singh’s Catalyst
PubMed Literature
Articles/Brochures
Product Documents (Note: Some products will not have analytical charts available.)
Safety Data Sheet (SDS)
Please select Language.
The requested SDS is not available.
Please Contact Us for more information.
Specifications
C of A & Other Certificates
Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
Sample C of A
This is a sample C of A and may not represent a recently manufactured lot of the product.
A sample C of A for this product is not available at this time.
Analytical Charts
Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
The requested analytical chart is not available. Sorry for the inconvenience.

![(2S)-N-[(1S)-1-(Hydroxydiphenylmethyl)-3-methylbutyl]-2-pyrrolidinecarboxamide (2S)-N-[(1S)-1-(Hydroxydiphenylmethyl)-3-methylbutyl]-2-pyrrolidinecarboxamide](/_ui/responsive/theme-tci/images/missing_product_zoom.jpg)

