Maximum quantity allowed is 999
Please select the quantity
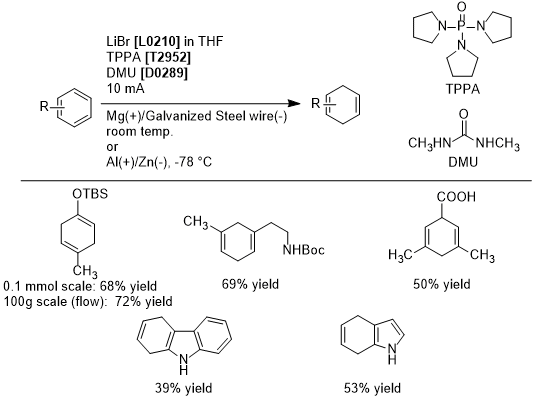
Sodium- and Ammonia-Free Birch Reduction by Electroreduction
Birch reduction is utilized in the partial hydrogenation of aromatic hydrocarbons, but has scale-up limitations due to requiring liquid ammonia and alkali metal at low temperatures and the generation of large quantities of hydrogen gas during quenching. To surmount this limitation, Baran et. al. have reported the electroreduction of aromatic compounds conducted with tripyrrolidinophosphine oxide (TPPA), 1,3-dimethylurea (DMU), and lithium bromide in THF, with magnesium as the anode, and galvanized steel wire as the cathode, to afford 1,4-dienes in good yields. This method has a high functional group tolerance, and can be applied for the removal of benzyl groups and for epoxide opening. A scaled-up Birch reduction in flow synthesis also provides 1,4-dienes in similar yields. In this way, electrochemical Birch reductions can be excepted to be utilized in both lab and at industrial scales.
Related Products
References
- Scalable and safe synthetic organic electroreduction inspired by Li-ion battery chemistry