Maximum quantity allowed is 999
Please select the quantity
CAS RN: 81290-20-2 | 제품번호: T1570
(Trifluoromethyl)trimethylsilane [Trifluoromethylating Reagent]
![(Trifluoromethyl)trimethylsilane [Trifluoromethylating Reagent] No-Image](/medias/T1570.jpg?context=bWFzdGVyfHJvb3R8NzU0MTB8aW1hZ2UvanBlZ3xhRGRsTDJoaVpTODRPVEl6TkRFM05qY3pOelU0TDFReE5UY3dMbXB3Wnd8YjdiMmNiNWZiODExMzE5ZDE3Mzg5MDVmNDYwOGIzYTM1OGQxYzY1YWZhZTlkNTg3OTczMmI0MmY2YzhhNTQzZg)
•본 제품의 재고는 일본 본사의 재고입니다. 주문시, 5일안에 실험실까지 도착됩니다.
•본건의 원가격은 한국 대리점의 예상 판매가격입니다.자세한 정보가 필요하시면 연락해 주십시오.( SEJIN CI Co., Ltd. (한국총대리점) 전화 : 02-2655-2480 이메일 : sales@sejinci.co.kr)
•보관 조건은 예고없이 변경 될 수 있습니다. 제품 보관 조건의 최신 자료는 홈페이지에 기재되어 있으니 양해 부탁드립니다.
•본건의 원가격은 한국 대리점의 예상 판매가격입니다.자세한 정보가 필요하시면 연락해 주십시오.( SEJIN CI Co., Ltd. (한국총대리점) 전화 : 02-2655-2480 이메일 : sales@sejinci.co.kr)
•보관 조건은 예고없이 변경 될 수 있습니다. 제품 보관 조건의 최신 자료는 홈페이지에 기재되어 있으니 양해 부탁드립니다.
| 제품번호 | T1570 |
| Purity/Analysis Method | >97.0%(GC) |
| M.F. / M.W. | C__4H__9F__3Si = 142.20 |
| 물리적 상태 (20 ℃) | Liquid |
| 보관 조건 | Room Temperature (Recommended in a cool and dark place, <15°C) |
| 불활성 가스 하에서 보관 | Store under inert gas |
| 피해야 할 조건 | Moisture Sensitive |
| CAS RN | 81290-20-2 |
| Reaxys-RN | 4241868 |
| PubChem Substance ID | 87577328 |
| SDBS | 19694 |
| Merck Index (14) | 9683 |
| MDL 번호 | MFCD00145454 |
규격표
| Appearance | Colorless to Light yellow clear liquid |
| Purity(GC) | min. 97.0 % |
물성치(참고치)
| bp | 55 °C |
| flp | -32 °C |
| 비중 | 0.96 |
| 굴절률 | 1.33 |
GHS
| 픽토그램 |

|
| 신호 워드 | Danger |
| 위험물 및 유해 등록 | H225 : Highly flammable liquid and vapor. |
| 주의 사항 | P501 : Dispose of contents/ container to an approved waste disposal plant. P240 : Ground/bond container and receiving equipment. P210 : Keep away from heat/sparks/open flames/hot surfaces. No smoking. P233 : Keep container tightly closed. P243 : Take precautionary measures against static discharge. P241 : Use explosion-proof electrical/ ventilating/ lighting/ equipment. P242 : Use only non-sparking tools. P280 : Wear protective gloves/ eye protection/ face protection. P370 + P378 : In case of fire: Use dry sand, dry chemical or alcohol-resistant foam to extinguish. P303 + P361 + P353 : IF ON SKIN (or hair): Take off immediately all contaminated clothing. Rinse skin with water/shower. P403 + P235 : Store in a well-ventilated place. Keep cool. |
법규 정보
운송 정보
| UN 번호 | UN1993 |
| 등급 | 3 |
| 포장 그룹 | II |
| HS 번호* | 2931.90-000 |
Application
Synthesis of Trifluoromethylketones from Weinreb Amides
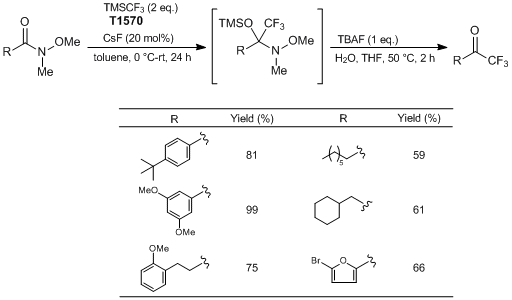
Typical Procedure:
To a 50 mL round bottom flask equipped with a stir bar is added CsF (0.1512 g, 1.0 mmol, 0.2 eq.). Toluene (2.5 mL) is added to the flask, followed by 4-(tert-butyl)-N-methoxy-N-methylbenzamide (1.11 g, 5.0 mmol, 1 eq.). The flask is sealed with a septum equipped with an inlet needle as an exit valve. The flask is cooled to 0 °C for 10 minutes. Once cooled, TMSCF3 (1.42 g, 10.0 mmol, 2 eq.) is added to the reaction mixture dropwise over a period of ≈ 10 minutes. After completion of addition, the reaction mixture is allowed to stir at 0 °C for 10 minutes. The cooling bath is removed and the reaction mixture is allowed to stir at room temperature overnight. (Upon reaching room temperature, the reaction occurs and is mildly exothermic and gas is evolved.) Reaction progress is monitored by 1H NMR.
Once complete conversion to the silylated intermediate is confirmed, water (5 mL) followed by TBAF (5 mL, 1 M in THF, 1 eq.) is added to the reaction flask. The flask is equipped with a reflux condenser, open to air. The contents are then heated to 50 °C, and allowed to stir at that temperature for 2 hours. Once cooled to room temperature, the reaction mixture is diluted with Et2O (≈ 30 mL), and transferred to a separatory funnel. The organic layer is washed with deionized water (3 × 30 mL), followed with a brine solution (1 × 30 mL). The organic layer is dried with Na2SO4 and the solvent is removed in vacuo by rotary evaporation to yield crude trifluoromethylketone. Further purification is accomplished by flash chromatography (hexane : EtOAc = 8 : 2) to produce the pure CF3 ketone as an orange solid (0.935 g, Y. 81%).
To a 50 mL round bottom flask equipped with a stir bar is added CsF (0.1512 g, 1.0 mmol, 0.2 eq.). Toluene (2.5 mL) is added to the flask, followed by 4-(tert-butyl)-N-methoxy-N-methylbenzamide (1.11 g, 5.0 mmol, 1 eq.). The flask is sealed with a septum equipped with an inlet needle as an exit valve. The flask is cooled to 0 °C for 10 minutes. Once cooled, TMSCF3 (1.42 g, 10.0 mmol, 2 eq.) is added to the reaction mixture dropwise over a period of ≈ 10 minutes. After completion of addition, the reaction mixture is allowed to stir at 0 °C for 10 minutes. The cooling bath is removed and the reaction mixture is allowed to stir at room temperature overnight. (Upon reaching room temperature, the reaction occurs and is mildly exothermic and gas is evolved.) Reaction progress is monitored by 1H NMR.
Once complete conversion to the silylated intermediate is confirmed, water (5 mL) followed by TBAF (5 mL, 1 M in THF, 1 eq.) is added to the reaction flask. The flask is equipped with a reflux condenser, open to air. The contents are then heated to 50 °C, and allowed to stir at that temperature for 2 hours. Once cooled to room temperature, the reaction mixture is diluted with Et2O (≈ 30 mL), and transferred to a separatory funnel. The organic layer is washed with deionized water (3 × 30 mL), followed with a brine solution (1 × 30 mL). The organic layer is dried with Na2SO4 and the solvent is removed in vacuo by rotary evaporation to yield crude trifluoromethylketone. Further purification is accomplished by flash chromatography (hexane : EtOAc = 8 : 2) to produce the pure CF3 ketone as an orange solid (0.935 g, Y. 81%).
References
Application
Nucleophilic Trifluoromethylation

Reference
Application
Difluorocarbene Precursor

Reference
- Synthesis of gem-difluorinated cyclopropanes and cyclopropenes: trifluoromethyltrimethylsilane as a difluorocarbene source
PubMed Literature
기사 / 브로셔
제품문서 (분석 차트를 제공하지 못할 수도 있으니 양해 바랍니다.)
SDS
언어를 선택하십시오.
요청한 SDS를 사용할 수 없습니다.
번거롭게 해드려 죄송하지만 이 양식 보다 문의 해주십시오.
규격표
시험성적서, 각종 증명서
롯트 번호를 입력하십시오.
잘못된 로트 번호가 입력되었습니다.
샘플 시험성적서
시험성적서 샘플을 다운로드할 수 있습니다. 샘플은 반드시 최신 로트의 결과를 나타내는 것은 아닙니다.
본 제품의 샘플시험성적서는 현재 준비되어 있지 않습니다.
분석 차트
롯트 번호를 입력하십시오.
잘못된 로트 번호가 입력되었습니다.
죄송합니다만 찾으시는 분석차트는 없습니다.

![(Trifluoromethyl)trimethylsilane [Trifluoromethylating Reagent] (Trifluoromethyl)trimethylsilane [Trifluoromethylating Reagent]](/_ui/responsive/theme-tci/images/missing_product_zoom.jpg)


