Maximum quantity allowed is 999
CAS RN: 2564-83-2 | 제품번호: T1560
2,2,6,6-Tetramethylpiperidine 1-Oxyl Free Radical

•본건의 원가격은 한국 대리점의 예상 판매가격입니다.자세한 정보가 필요하시면 연락해 주십시오.( SEJIN CI Co., Ltd. (한국총대리점) 전화 : 02-2655-2480 이메일 : sales@sejinci.co.kr)
•보관 조건은 예고없이 변경 될 수 있습니다. 제품 보관 조건의 최신 자료는 홈페이지에 기재되어 있으니 양해 부탁드립니다.
| 제품번호 | T1560 |
| Purity/Analysis Method | >98.0%(GC)(T) |
| M.F. / M.W. | C__9H__1__8NO = 156.25 |
| 물리적 상태 (20 ℃) | Solid |
| 보관 조건 | Refrigerated (0-10°C) |
| 불활성 가스 하에서 보관 | Store under inert gas |
| 피해야 할 조건 | Air Sensitive,Heat Sensitive |
| CAS RN | 2564-83-2 |
| Reaxys-RN | 1422418 |
| PubChem Substance ID | 87577318 |
| Merck Index (14) | 9140 |
| MDL 번호 | MFCD00009599 |
| Appearance | Orange to Brown powder to crystal |
| Purity(GC) | min. 98.0 % |
| Purity(Iodometric Titration) | min. 98.0 % |
| Solubility in Methanol | almost transparency |
| mp | 39 °C |
| flp | 67 °C |
| 용해성 (용해) | Methanol |
| 픽토그램 |

|
| 신호 워드 | Danger |
| 위험물 및 유해 등록 | H314 : Causes severe skin burns and eye damage. |
| 주의 사항 | P501 : Dispose of contents/ container to an approved waste disposal plant. P260 : Do not breathe dusts or mists. P264 : Wash skin thoroughly after handling. P280 : Wear protective gloves/ protective clothing/ eye protection/ face protection. P303 + P361 + P353 : IF ON SKIN (or hair): Take off immediately all contaminated clothing. Rinse skin with water/shower. P301 + P330 + P331 : IF SWALLOWED: Rinse mouth. Do NOT induce vomiting. P363 : Wash contaminated clothing before reuse. P304 + P340 + P310 : IF INHALED: Remove person to fresh air and keep comfortable for breathing. Immediately call a POISON CENTER/doctor. P305 + P351 + P338 + P310 : IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. Immediately call a POISON CENTER/doctor. P405 : Store locked up. |
| RTECS # | TN8991900 |
| UN 번호 | UN3263 |
| 등급 | 8 |
| 포장 그룹 | II |
| HS 번호* | 2933.39-000 |

-
Used Chemicals
-
Procedure
-
To a solution of 1-naphthalenemethanol (306 mg, 2.0 mmol) in dichloromethane (2 mL, 1.0 mol/L) was added TEMPO (31.2 mg, 0.20 mmol), PhI(OAc)2 (709mg, 2.2 mmol) and the mixture was stirred at room temperature for 4 hours. Dichloromethane (12.5 mL), saturated aqueous sodium thiosulfate solution (12.5 mL) was added and the mixture was stirred for 30 minutes. The organic layer was washed with saturated aqueous sodium bicarbonate solution (10 mL), brine (10 mL) and the organic layer was dried over sodium sulfate and filtered. The solvent was removed under reduced pressure and the residue was purified by column chromatography (ethyl acetate:hexane = 0:100 - 3:97 on silica gel) to give 1-naphthaldehyde as a yellow liquid (291 mg, 93%).
-
Experimenter’s Comments
-
The reaction mixture was monitored by TLC (ethyl acetate:hexane = 1:9, Rf = 0.50).
-
Analytical Data
-
1-Naphthaldehyde
1H NMR (400 MHz, CDCl3); δ 10.41 (s, 1H), 9.26 (d, J = 8.9 Hz, 1H), 8.11 (d, J = 8.4 Hz, 1H), 8.01 (d, J = 6.8 Hz, 1H), 7.93 (d, J = 8.9 Hz, 1H), 7.74-7.57 (m, 3H).
-
Lead Reference
-
- 2-(Hydroxyimino)aldehydes: Photo- and Physicochemical Properties of a Versatile Functional Group for Monomer Design
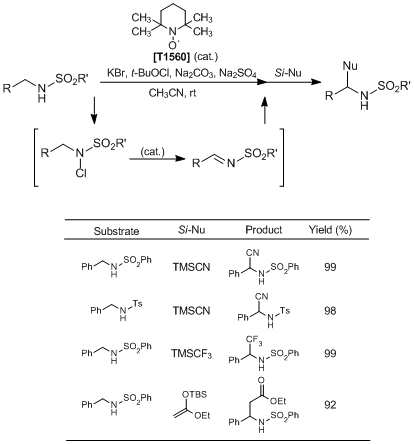
To a solution of N-benzylbenzenesulfonamide (61.8 mg, 0.25 mmol), KBr (14.9 mg, 0.125 mmol), and Na2SO4 (71.0 mg, 0.50 mmol) in MeCN (1.5 mL) is added t-BuOCl (33.9 µL, 0.30 mmol) at room temperature, and the mixture is stirred at room temperature for 1 h. Then, TEMPO (7.8 mg, 0.050 mmol) is added and stirred at room temperature for 30 min. To the solution is added Na2CO3 (26.5 mg, 0.25 mmol), and the mixture is stirred at room temperature for 20 h. Subsequently, TMSCN (93.8 mL, 0.75 mmol) and LiClO4 (2.7 mg, 0.025 mmol) are added to the reaction mixture, and further stirred at room temperature for 22 h. Saturated Na2SO3 aqueous solution (10 mL) is added to the mixture, and the product is extracted with AcOEt (15 mL×3). The organic phase is washed with brine and dried over Na2SO4. The organic phase is concentrated under reduced pressure, and the crude product is purified by column chromatography (hexane : AcOEt = 3 : 1) to give the desired product (67.4 mg, Y. 99%).
References
References
- Indirect electrooxidation of alcohols
- Selective oxidation of monosaccharide derivatives
- Alcohol oxidation in an N-oxoammonium salts-NaBrO2 system
- Alcohol oxidation using organic oxoammonium salts
- Alcohol oxidation with oxygen and cupric ion
- Review
기사 / 브로셔
[Research Articles] Nitroxyl-radical-catalyzed Oxidative Coupling of Amides with Silylated Nucleophiles
SDS
요청한 SDS를 사용할 수 없습니다.
번거롭게 해드려 죄송하지만 이 양식 보다 문의 해주십시오.
규격표
시험성적서, 각종 증명서
샘플 시험성적서
본 제품의 샘플시험성적서는 현재 준비되어 있지 않습니다.
분석 차트
죄송합니다만 찾으시는 분석차트는 없습니다.





