Maximum quantity allowed is 999
CAS RN: 19350-66-4 | 제품번호: B6316
3,5-Bis(ethoxycarbonyl)-2,6-dimethyl-1,4-dihydropyridine-4-carboxylic Acid

•본건의 원가격은 한국 대리점의 예상 판매가격입니다.자세한 정보가 필요하시면 연락해 주십시오.( SEJIN CI Co., Ltd. (한국총대리점) 전화 : 02-2655-2480 이메일 : sales@sejinci.co.kr)
•보관 조건은 예고없이 변경 될 수 있습니다. 제품 보관 조건의 최신 자료는 홈페이지에 기재되어 있으니 양해 부탁드립니다.
| 제품번호 | B6316 |
| Purity/Analysis Method | >95.0%(T)(HPLC) |
| M.F. / M.W. | C__1__4H__1__9NO__6 = 297.31 |
| 물리적 상태 (20 ℃) | Solid |
| 보관 조건 | Room Temperature (Recommended in a cool and dark place, <15°C) |
| 불활성 가스 하에서 보관 | Store under inert gas |
| 피해야 할 조건 | Air Sensitive |
| 용기 | 1G-Glass Bottle with Plastic Insert (이미지 보기) |
| CAS RN | 19350-66-4 |
| Reaxys-RN | 489397 |
| PubChem Substance ID | 468591034 |
| MDL 번호 | MFCD00417306 |
| Appearance | White to Light yellow powder to crystal |
| Purity(HPLC) | min. 95.0 area% |
| Purity(Neutralization titration) | min. 95.0 % |
| NMR | confirm to structure |
| mp | 220 °C |
| 픽토그램 |

|
| 신호 워드 | Warning |
| 위험물 및 유해 등록 | H315 : Causes skin irritation. H319 : Causes serious eye irritation. |
| 주의 사항 | P264 : Wash skin thoroughly after handling. P280 : Wear protective gloves/ eye protection/ face protection. P302 + P352 : IF ON SKIN: Wash with plenty of water. P337 + P313 : If eye irritation persists: Get medical advice/ attention. P305 + P351 + P338 : IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. P362 + P364 : Take off contaminated clothing and wash it before reuse. P332 + P313 : If skin irritation occurs: Get medical advice/ attention. |
| HS 번호* | 2933.39-000 |
-
Used Chemicals
-
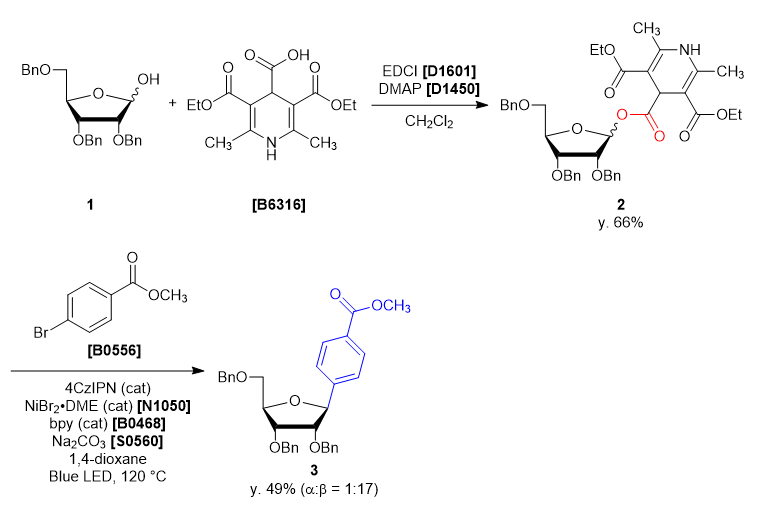
- 3,5-Bis(ethoxycarbonyl)-2,6-dimethyl-1,4-dihydropyridine-4-carboxylic Acid [B6316]
- 2,3,5-Tri-O-benzyl-α/β-D-ribofuranose (1)
- 4-Dimethylaminopyridine (= DMAP) [D1450]
- 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide Hydrochloride (= EDCI) [D1601]
- Dichloromethane
- Sodium Carbonate [S0560]
- 1,2,3,5-Tetrakis(carbazol-9-yl)-4,6-dicyanobenzene (= 4CzIPN)
- Methyl 4-Bromobenzoate [B0556]
- 2,2'-Bipyridyl (= bpy) [B0468]
- Nickel(II) Bromide Ethylene Glycol Dimethyl Ether Complex (= NiBr2・DME) [N1050]
- 1,4-Dioxane
-
Procedure
-
A four-neck round bottom flask was charged with 2,3,5-tri-O-benzyl-α/β-D-ribofuranose (1) (2.165 g, 5.15 mmol, 1 eq) and dichloromethane (26 mL). The solution was cooled under 5 ˚C, then DMAP (0.063 g, 0.51 mmol, 0.1 eq), EDCI (1.38 g, 7.21 mmol, 1.4 eq), 3,5-bis(ethoxycarbonyl)-2,6-dimethyl-1,4-dihydropyridine-4-carboxylic acid (1.684 g, 5.66 mmol, 1.1 eq) was added. The reaction mixture was allowed to warm to ambient temperature and stirred for 46 h. After the reaction, the reaction mixture was quenched with ion-exchanged water (35 mL). The solution was transferred into a separatory funnel, and the aqueous layer was extracted with dichloromethane (10 mL, twice). The combined organic layers were washed with brine, dried over sodium sulfate (10 g) for about 30 minutes and then filtered. The solvent was removed in vacuo, giving crude as a blown oil (3.91 g). The crude was purified by silica gel column chromatography (hexane:ethyl acetate = 1:1, Rf = 0.45) to give compound 2 as a light yellow oil (2.99 g, y. 66%).
30 mL sealed vessel was charged with 4CzIPN (0.0158 g, 0.020 mmol, 0.1 eq), methyl 4-bromobenzoate (0.0858 g, 0.399 mmol, 2.0 eq), sodium carbonate (0.0443 g, 0.418 mmol, 2.1 eq), 2,2’-bipyridine (0.0086 g, 0.055 mmol, 0.28 eq) at rt under N2. A solution of NiBr2・DME (0.012 g, 0.040 mmol, 0.20 eq) in 1,4-dioxane (5 mL) and the solution of 1 (0.14 g, 0.20 mmol, 1.0 eq) in 1,4-dioxane (5 mL) were added to the sealed vessel. The mixture was placed in a preheated oil bath whose temperature was set to 120 ˚C, and placed at a distance of 2-3 cm from Blue LED lamp. After irradiation for 46 h, the reaction mixture was cooled to room temperature and filtered through Celite pad (1 cm). The solvent was removed in vacuo and the crude was given as a yellow oil (0.23 g). The crude was purified by silica gel column chromatography (hexane:ethyl acetate = 10:2, Rf = 0.30) to give compound 3 as a colorless oil (0.053 g, y. 49%). -
-
Experimenter’s Comments
-
- NiBr2・DME was weighed in a nitrogen-filled glove box and dissolved in 1,4-dioxane completely using a sonication.
- 1,4-Dioxane was degassed with nitrogen for 30 min before use.
- Irradiation of visible light was performed with Kessil A160WE Tuna Blue 40W x 2.
- The reaction mixture was monitored by 1H NMR and UPLC.
- The α/β selectivity of 3 was 1:17.
-
Analytical Data
-
Compound 3
1H NMR (270 MHz, CDCl3); δ 7.96 (d, J = 8.2 Hz, 2H), 7.46 (d, J = 8.2 Hz, 2H), 7.40-7.30 (m, 11H), 7.26-7.23 (m, 2H), 7.18-7.15 (m, 2H), 5.05 (d, J = 6.9 Hz, 1H), 4.71-4.44 (m, 6H), 4.39-4.34 (m, 1H), 4.01 (dd, J = 5.3, 3.3 Hz, 1H), 3.92 (s, 3H), 3.77 (dd, J = 6.9, 5.3 Hz, 1H), 3.65 (qd, J = 10.6, 3.9 Hz, 2H).
-
Lead Reference
-
- Diastereoselective Synthesis of Aryl C-Glycosides from Glycosyl Esters via C-O Bond Homolysis
-
Other Reference
-
- Highly stereoselective synthesis of aryl/heteroaryl-C-nucleosides via the merger of photoredox and nickel catalysis
기사 / 브로셔
SDS
요청한 SDS를 사용할 수 없습니다.
번거롭게 해드려 죄송하지만 이 양식 보다 문의 해주십시오.
규격표
시험성적서, 각종 증명서
샘플 시험성적서
본 제품의 샘플시험성적서는 현재 준비되어 있지 않습니다.
분석 차트
죄송합니다만 찾으시는 분석차트는 없습니다.




