N-Heterocyclic carbene (NHC) is a cyclic carbene species with two neighboring nitrogen atoms. NHC was discovered by Wanzlick
et al. in 1968, and in 1991, it was isolated and structure determined by Arduengo
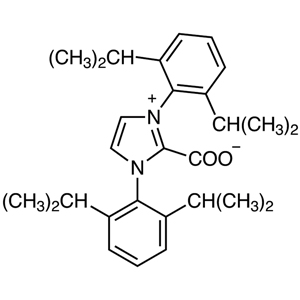
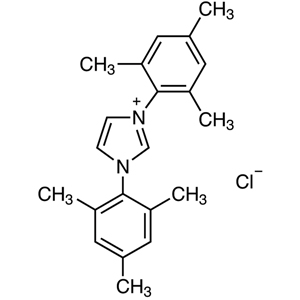
et al. So, for their achievements, NHC is also called as the Wanzlick-Arduengo type carbene. Generally, NHC is hard to isolate as a single carbene monomer because it easily dimerizes or reacts with water to decompose. However, NHC is conformationally stabilized by introducing bulky substituents on the nitrogen atoms of NHC. NHC substituted by a mesityl groups or 2,6-diisopropylphenyl groups is commonly used as a ligand for organometallic complexes.

A characteristic property of NHC ligands is their high coordinating ability caused by their bulkiness and strong electron-donating property. Their effect is stronger relative to trialkylphosphines, and allows the formation of metal-NHC complexes by ligand exchange reactions of metal-phosphine complexes with NHC ligands. In this way, NHC ligands strongly form complexes with some metals, and also produce coordinative unsaturation species by pushing out a trans position ligand coordinated with the metal center. Therefore, metal complexes coordinated by NHC ligands are highly active species but chemically stable and easy to handle, so they are expected to have a high turnover frequency.
Metal-NHC complexes can be prepared by a complex-forming reaction of metal complexes having anionic ligands such as acetoxy ions with NHC which was previously prepared from imidazolium salts and bases. They can be also prepared via the carbene-exchange reaction of silver-carbene complexes prepared from silver(I) oxide and imidazolium salts. This method is effective to use when bases are unavailable for preparing NHC. Metal-NHC complexes have been used for various chemical transformations such as cross-coupling reactions, cycloaddition reactions and C-H bond activation reactions since they have been successfully applied in metathesis reactions. In addition, NHC is used as an organocatalyst for benzoin condensations and acyloin condensations via the umpolung process.
References
- 1) S. P. Nolan, N-Heterocyclic Carbenes in Synthesis Wiley-VCH, Weinheim, 2006.
- 2) J. F. Hartwig, Organotransition Metal Chemistry: From Bonding to Catalysis Univ Science Books, 2009.