Maximum quantity allowed is 999
Please select the quantity
CAS RN: 68986-76-5 | Product Number: C2312
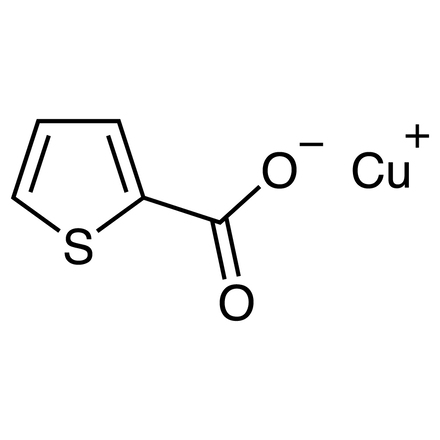
Copper(I) 2-Thiophenecarboxylate
Purity:
Synonyms:
- 2-Thiophenecarboxylic Acid Copper(I) Salt
- (2-Thiophenecarboxylato)copper(I)
- CuTC
Product Documents:
* Please contact our distributor of SEJIN CI Co., Ltd.
if you would like to purchase TCI products. The above prices do not include freight cost, customs charge and other charges to the destination. SEJIN CI Co., Ltd. (Phone: 02-2655-2480 / email:
sales@sejinci.co.kr)
* The storage conditions are subject to change without notice.
* The storage conditions are subject to change without notice.
| Product Number | C2312 |
| Molecular Formula / Molecular Weight | C__5H__3CuO__2S = 190.68 |
| Physical State (20 deg.C) | Solid |
| Storage Temperature | Room Temperature (Recommended in a cool and dark place, <15°C) |
| Store Under Inert Gas | Store under inert gas |
| Condition to Avoid | Moisture Sensitive |
| Packaging and Container | 1G-Glass Bottle with Plastic Insert (View image) |
| CAS RN | 68986-76-5 |
| PubChem Substance ID | 253660329 |
| Merck Index (14) | 2521 |
| MDL Number | MFCD02183524 |
Specifications
| Elemental analysis(Carbon) | 25.00 to 35.00 % |
| Appearance | Orange to Brown to Dark red powder to crystal |
| Content(Copper) | 30.0 to 40.0 % |
Properties (reference)
GHS
| Pictogram |

|
| Signal Word | Warning |
| Hazard Statements | H315 : Causes skin irritation. H319 : Causes serious eye irritation. |
| Precautionary Statements | P264 : Wash skin thoroughly after handling. P280 : Wear protective gloves/ eye protection/ face protection. P302 + P352 : IF ON SKIN: Wash with plenty of water. P337 + P313 : If eye irritation persists: Get medical advice/ attention. P305 + P351 + P338 : IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. P362 + P364 : Take off contaminated clothing and wash it before reuse. P332 + P313 : If skin irritation occurs: Get medical advice/ attention. |
Related Laws:
Transport Information:
| H.S.code* | 2934.99-000 |
Application
Copper/photoredox-catalyzed Decarboxylative sp3 C–N Coupling Reaction of N-Heteroaromatics
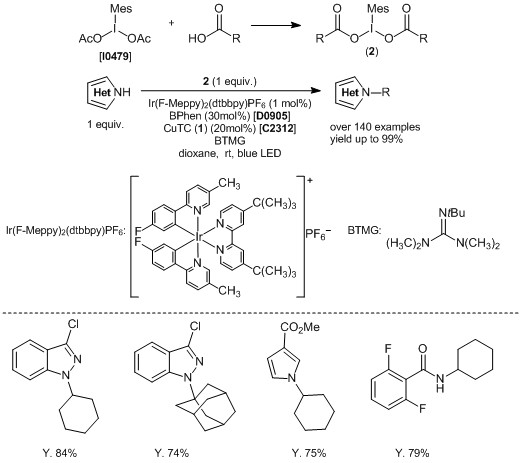
Preparation of Iodomesitylene Dicarboxylates: Iodomesitylene diacetate (10 mmol), cyclohexyl carboxylic acid (20.5 mmol), and toluene (200 mL) are placed in flask. The solvent is removed by a rotary evaporator at 55 °C. This step is repeated with toluene (100 mL) as needed. The residue is concentrated in vacuo, to provide iodomesitylene dicarboxylates that can be directly used in the decarboxylative sp3 C–N coupling reactions without further purification. sp3 C–N Coupling Reaction: Ir(F-Meppy)2(dtbbpy)PF6 (4.9 mg, 5.0 µmol), copper(I) 2-Thiophenecarboxylate (19 mg, 0.10 mmol), bathophenanthroline (50 mg, 0.15 mmol), 2-tert-butyl-1,1,3,3-tetramethylguanidine (0.21 mL, 171 mg, 1.0 mmol), 3-chloro-1H-indazole (76 mg, 0.50 mmol), iodomesitylene dicyclohexanecarboxylate (500 mg, 1.0 mmol), and 1,4-dioxane(12 mL) is placed in vial. The solution is sonicated until it becomes homogeneous and is then degassed with nitrogen. The reaction is stirred and irradiated using two 34-W blue LED lamps for 1 h. After the reaction has completed, the reaction mixture is diluted with water and EtOAc. The aqueous layer is extracted with EtOAc. The combined organic layers are washed with brine, dried over Na2SO4, filtered and concentrated in vacuo. The residue is purified by flash chromatography (30:1 hexane/EtOAc) on silica gel to afford the title compound (99 mg, 84% yield, >20:1 r.r.) as a colorless oil.
References
- Decarboxylative sp3 C–N coupling via dual copper and photoredox catalysis
Application
Copper (I) Complex Promoting Various Coupling Reactions

References
- 1)G. D. Allred, L. S. Liebeskind, J. Am. Chem. Soc. 1996, 118, 2748.

- 2)S. Zhang, D. Zhang, L. S. Liebeskind, J. Org. Chem. 1997, 62, 2312.

- 3)L. S. Liebeskind, J. Srogl, J. Am. Chem. Soc. 2000, 122, 11260.

Application
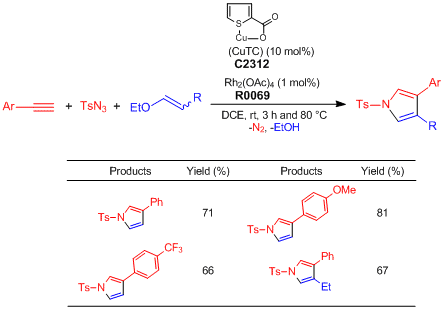
One-pot Synthesis of Pyrroles from Terminal Alkynes, N-Sulfonyl Azides, and Alkenyl Alkyl Ethers
Typical Procedure:
Alkyne (0.2 mmol), TsN3 (1.0 eq.), alkenyl alkyl ether (3.0 eq.), CuTC (10.0 mol%), Rh2(OAc)4 (1.0 mol%), and DCE (0.2 M) are added to an oven-dried test tube under nitrogen atmosphere. The reaction mixture is stirred at ambient temperature for 3 h and then, heated at 80 °C for 4–13 h. After the reaction mixture is filtered through short path of celite with CH2Cl2, the filtrate is concentrated under reduced pressure. The residue is purified by silica gel column chromatography (ethyl acetate–hexane) to give pyrrole derivatives (Y. 65–81%).
Alkyne (0.2 mmol), TsN3 (1.0 eq.), alkenyl alkyl ether (3.0 eq.), CuTC (10.0 mol%), Rh2(OAc)4 (1.0 mol%), and DCE (0.2 M) are added to an oven-dried test tube under nitrogen atmosphere. The reaction mixture is stirred at ambient temperature for 3 h and then, heated at 80 °C for 4–13 h. After the reaction mixture is filtered through short path of celite with CH2Cl2, the filtrate is concentrated under reduced pressure. The residue is purified by silica gel column chromatography (ethyl acetate–hexane) to give pyrrole derivatives (Y. 65–81%).
References
PubMed Literature
Articles/Brochures
TCIMAIL
[TCIMAIL No.166] Useful Copper Reagent for Coupling Reactions[Product Highlights] Copper/photoredox-catalyzed Decarboxylative sp3 C–N Coupling Reaction of N-Heteroaromatics
[Product Highlights] Copper (I) Complex Promoting Various Coupling Reactions
[Research Articles] One-pot Synthesis of Pyrroles from Terminal Alkynes, N-Sulfonyl Azides, and Alkenyl Alkyl Ethers
Product Documents (Note: Some products will not have analytical charts available.)
Safety Data Sheet (SDS)
Please select Language.
The requested SDS is not available.
Please Contact Us for more information.
Specifications
C of A & Other Certificates
Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
Sample C of A
This is a sample C of A and may not represent a recently manufactured lot of the product.
A sample C of A for this product is not available at this time.
Analytical Charts
Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
The requested analytical chart is not available. Sorry for the inconvenience.




