Maximum quantity allowed is 999
CAS RN: 71432-55-8 | Product Number: B4178
O-tert-Butyl-N,N'-diisopropylisourea

Purity: >98.0%(T)
- 2-tert-Butyl-1,3-diisopropylisourea
| Size | Unit Price | Same Day | 2-3 Business Days | Other Lead Time |
Shipping Information

|
|---|---|---|---|---|---|
| 1G |
₩155,400
|
2 | 22 | Contact Us | |
| 5G |
₩521,700
|
≥40 | ≥100 | Contact Us |
* The storage conditions are subject to change without notice.
| Product Number | B4178 |
| Purity / Analysis Method | >98.0%(T) |
| Molecular Formula / Molecular Weight | C__1__1H__2__4N__2O = 200.33 |
| Physical State (20 deg.C) | Liquid |
| Storage Temperature | Refrigerated (0-10°C) |
| Store Under Inert Gas | Store under inert gas |
| Condition to Avoid | Moisture Sensitive,Heat Sensitive |
| CAS RN | 71432-55-8 |
| Reaxys Registry Number | 1764410 |
| PubChem Substance ID | 253660124 |
| MDL Number | MFCD06657672 |
| Appearance | Colorless to Almost colorless clear liquid |
| Purity(Nonaqueous Titration) | min. 98.0 % |
| Boiling Point | 61 °C/10 mmHg |
| Flash point | 135 °C |
| Specific Gravity (20/20) | 0.84 |
| Refractive Index | 1.43 |
| H.S.code* | 2925.29-000 |
-
Used Chemicals
-
- Boc-Ser-OH [B1637]
- O-tert-Butyl-N,N'-diisopropylisourea [B4178]
- Dichloromethane
-
Procedure
-
To a solution of Boc-Ser-OH (0.94 g, 4.6 mmol) in dichloromethane (10 mL) O-tert-Butyl-N,N'-diisopropylisourea (3.3 mL, 14.0 mmol) was added dropwise over 5 min at 3 °C then stirred overnight at room temperature under nitrogen atmosphere. To the reaction mixture hexane (10 mL) was added then filtered through celite and the solvent was removed under reduced pressure. The residue was purified by column chromatography (1:1 ethyl acetate / hexane on SiO2), giving Boc-Ser-OtBu as a white solid (0.59 g, 50% yield).
-
Experimenter's Comments
-
The reaction mixture was monitored by TLC (hexane : ethyl acetate = 1:1, Rf = 0.60).
-
Analytical Data(Boc-Ser-OtBu)
-
1H NMR (400 MHz, CDCl3); δ 5.57–5.30 (m, 1H), 4.36–4.15 (m, 1H), 3.97–3.79 (m, 2H), 2.57–2.34 (m, 1H), 1.51-1.41 (m, 18H).
13C NMR (101 MHz, CDCl3); δ 169.9 (2 C), 82.8, 80.3, 64.3, 56.5, 28.4 (3 C), 28.1 (3 C).
-
Lead Reference
-
- L-Type amino acid transporter 1 activity of 1,2,3-triazolyl analogs of L-histidine and L-tryptophan
-
Other References
-
- Esterification and Alkylation Reactions Employing Isoureas
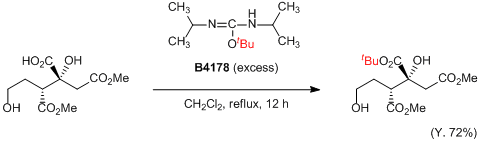
The starting material (310 mg, 0.87 mmol) in CH2Cl2 (15 mL) is allowed to react with O-tert-butyl-N,N’-diisopropylisourea (2 mL, excess) and the mixture is stirred under reflux for 12 h. Rotary evaporation and chromatography (hexane : Et2O = 1 : 1) gives the desired tert-butyl ester (260 mg, 0.63 mmol, Y. 72%) as a colorless oil.
References
- Total synthesis of citrafungin A
Articles/Brochures
[TCI Practical Example] tert-Butyl Esterification Utilizing the Isourea Derivative
Safety Data Sheet (SDS)
The requested SDS is not available.
Please Contact Us for more information.
Specifications
C of A & Other Certificates
Sample C of A
A sample C of A for this product is not available at this time.
Analytical Charts
The requested analytical chart is not available. Sorry for the inconvenience.



