Maximum quantity allowed is 999
Bioisostere is defined as substituents or substructures with similar biological properties and chemical and physical similarities.1,2) This is an established strategy in medicinal chemistry for the design and discovery of new compounds.3) In fact, angiotensin II receptor antagonists for the treatment of hypertension were developed by the introduction of tetrazole structure as carboxyl group bioisostere.4)
Recently, in addition to classical bioisosteres for carboxyl groups and amide bonds, bioisosteres corresponding to heterocycles and benzene rings have been developed and utilized. Spiro-building blocks containing small rings such as oxetane and azetidine have been used as bioisosteres for morpholine and piperazine, respectively.5,6) Cubane, bicyclo[1.1.1]pentane, bicyclo[2.1.1]hexane, and bicyclo[3.1 .1]heptane structures have been used as bioisosteres of benzene rings.7,8) Especially, disubstituted bicyclo[1.1.1]pentanes have been used as para-substituted benzenes,9) and bicyclo[2.1.1]hexane and bicyclo[3.1.1]heptane structures have been used as meta-substituted benzene.10,11) The introduction of these structures is expected to improve water solubility and pharmacokinetics by breaking the planarity due to the improvement of Fsp3.
Products
Morpholine Bioisostere
Piperazine Bioisostere
Benzene Bioisosteres
para-Disubstituted Benzene Bioisosteres
- M3149
- 3-(Methoxycarbonyl)bicyclo[1.1.1]pentane-1-carboxylic Acid
- A3655
- Methyl 3-Aminobicyclo[1.1.1]pentane-1-carboxylate Hydrochloride
- D6184
- 3-(Boc-amino)bicyclo[1.1.1]pentane-1-carboxylic Acid
- P3127
- Potassium Trifluoro[3-(methoxycarbonyl)bicyclo[1.1.1]pentan-1-yl]borate
- B6522
- Bicyclo[1.1.1]pentane-1,3-dicarboxylic Acid
- M3466
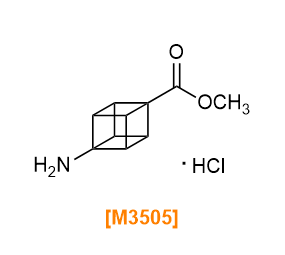
- 4-(Methoxycarbonyl)cubane-1-carboxylic Acid
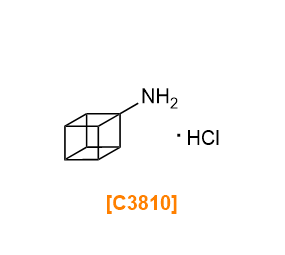
- M3505
- Methyl 4-Amino-1-cubanecarboxylate Hydrochloride
- B6415
- 4-(Boc-amino)-1-cubanecarboxylic Acid
meta-Disubstituted Benzene Bioisosteres
- M3703
- 4-(Methoxycarbonyl)bicyclo[2.1.1]hexane-1-carboxylic Acid
- M3705
- Methyl 4-Aminobicyclo[2.1.1]hexane-1-carboxylate Hydrochloride
- B6609
- 4-(Boc-amino)bicyclo[2.1.1]hexane-1-carboxylic Acid
- B6610
- tert-Butyl (4-Aminobicyclo[2.1.1]hexan-1-yl)carbamate
- P3128
- Potassium Trifluoro[3-(methoxycarbonyl)bicyclo[1.1.1]pentan-1-yl]borate
- M3701
- 5-(Methoxycarbonyl)bicyclo[3.1.1]heptane-1-carboxylic Acid
- M3751
- Methyl 5-Aminobicyclo[3.1.1]heptane-1-carboxylate
- B6607
- 5-(Boc-amino)bicyclo[3.1.1]heptane-1-carboxylic Acid
- B6608
- 5-(Boc-amino)bicyclo[3.1.1]heptane-1-amine
- P3129
- Potassium Trifluoro[5-(methoxycarbonyl)bicyclo[3.1.1]heptan-1-yl]borate
Product Brochures
References
- 1) Isosterism and molecular modification in drug design
- 2) Bioisosterism: A Rational Approach in Drug Design Cycloaddition
- 3) Applications of Bioisosteres in the Design of Biologically Active Compounds
- 4) Nonpeptide Angiotensin II Receptor Antagonists: The Next Generation in Antihypertensive Therapy
- 5) Spirocyclic Oxetanes: Synthesis and Properties
- 6) 2,6-Diazaspiro[3.3]heptanes: Synthesis and Application in Pd-Catalyzed Aryl Amination Reactions
- 7) Strain-Release Heteroatom Functionalization: Development, Scope, and Stereospecificity
- 8) Cubanes in Medicinal Chemistry
- 9) Bioisosteres of the Phenyl Ring: Recent Strategic Applications in Lead Optimization and Drug Design
- 10) Saturated bioisosteres of benzene: where to go next?
- 11) Practical and Facile Access to Bicyclo[3.1.1]heptanes: Potent Bioisosteres of meta-Substituted Benzenes Cycloaddition


![2-Oxa-6-azaspiro[3.3]heptane](https://www.tcichemicals.com/assets/cms-images/bioisosteres_O0466.png)
![2-Boc-2,6-diazaspiro[3.3]heptane](https://www.tcichemicals.com/assets/cms-images/bioisosteres_B5032.png)
![Bicyclo[1.1.1]pentan-1-amine Hydrochloride](https://www.tcichemicals.com/assets/cms-images/bioisosteres_B5479.png)
![Bicyclo[1.1.1]pentane-1-carboxylic Acid](https://www.tcichemicals.com/assets/cms-images/bioisosteres_B6619.png)
![2-Oxa-6-azaspiro[3.3]heptane](https://www.tcichemicals.com/assets/cms-images/bioisosteres_C3808.png)
![3-(Methoxycarbonyl)bicyclo[1.1.1]pentane-1-carboxylic Acid](https://www.tcichemicals.com/assets/cms-images/bioisosteres_M3149.png)
![Methyl 3-Aminobicyclo[1.1.1]pentane-1-carboxylate Hydrochloride](https://www.tcichemicals.com/assets/cms-images/bioisosteres_A3655.png)
![3-(Boc-amino)bicyclo[1.1.1]pentane-1-carboxylic Acid](https://www.tcichemicals.com/assets/cms-images/bioisosteres_D6184.png)
![Potassium Trifluoro[3-(methoxycarbonyl)bicyclo[1.1.1]pentan-1-yl]borate](https://www.tcichemicals.com/assets/cms-images/bioisosteres_P3127.png)
![Bicyclo[1.1.1]pentane-1,3-dicarboxylic Acid](https://www.tcichemicals.com/assets/cms-images/bioisosteres_B6522.png)


![4-(Methoxycarbonyl)bicyclo[2.1.1]hexane-1-carboxylic Acid](https://www.tcichemicals.com/assets/cms-images/bioisosteres_M3703.png)
![3-(Methoxycarbonyl)bicyclo[1.1.1]pentane-1-carboxylic Acid](https://www.tcichemicals.com/assets/cms-images/bioisosteres_M3705.png)
![4-(Boc-amino)bicyclo[2.1.1]hexane-1-carboxylic Acid](https://www.tcichemicals.com/assets/cms-images/bioisosteres_B6609.png)
![tert-Butyl (4-Aminobicyclo[2.1.1]hexan-1-yl)carbamate](https://www.tcichemicals.com/assets/cms-images/bioisosteres_B6610.png)
![Potassium Trifluoro[4-(methoxycarbonyl)bicyclo[2.1.1]hexan-1-yl]borate](https://www.tcichemicals.com/assets/cms-images/bioisosteres_P3128.png)
![5-(Methoxycarbonyl)bicyclo[3.1.1]heptane-1-carboxylic Acid](https://www.tcichemicals.com/assets/cms-images/bioisosteres_M3701.png)
![5-(Boc-amino)bicyclo[3.1.1]heptane-1-carboxylic Acid](https://www.tcichemicals.com/assets/cms-images/bioisosteres_M3751.png)
![5-(Boc-amino)bicyclo[3.1.1]heptane-1-carboxylic Acid](https://www.tcichemicals.com/assets/cms-images/bioisosteres_B6607.png)
![5-(Boc-amino)bicyclo[3.1.1]heptane-1-amine](https://www.tcichemicals.com/assets/cms-images/bioisosteres_B6608.png)
![Potassium Trifluoro[5-(methoxycarbonyl)bicyclo[3.1.1]heptan-1-yl]borate](https://www.tcichemicals.com/assets/cms-images/bioisosteres_P3129.png)