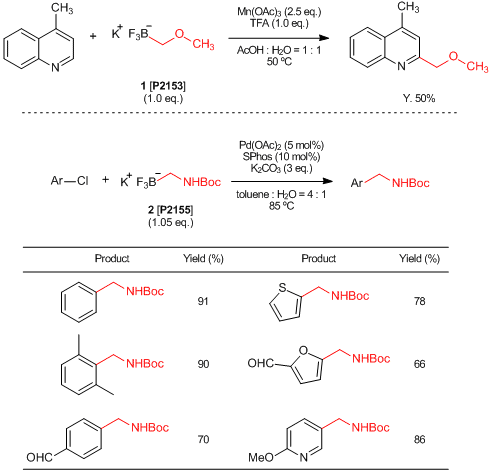
Organoboronic acids are commonly used reactants for Suzuki-Miyaura cross-coupling reactions. Generally, aryl boronic acids and alkenyl boronic acids are chemically stable and easy to treat while alkyl boronic acids are less stable and some of them are difficult to synthesize. Trifluoroborates are one of the organic boron compounds which are more electrically and chemically stable due to full substitution on their boron atom with fluorine atoms. For instance,
potassium (methoxymethyl)trifluoroborate (1) and
potassium (N-Boc-aminomethyl)trifluoroborate (2) are stable and solid trifluoroborate derivatives which can be used for introducing methoxymethyl
1) and aminomethyl
2) groups by transition-metal catalyzed cross-coupling reactions.