Published TCIMAIL newest issue No.196
Maximum quantity allowed is 999
Please select the quantity
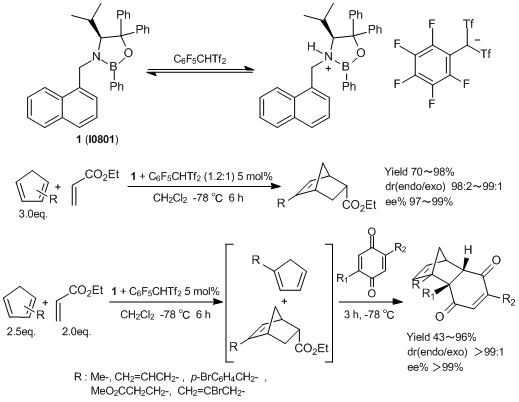
An Efficient Asymmetric Organocatalyst for Regio- and Enantio-selective Diels-Alder Reactions
(S)-4-isopropyl-3-(1-naphthylmethyl)-2,5,5-triphenyl-1,3,2-oxazaborolidine (1) is an asymmetric organocatalyst developed by Yamamoto et al. 1 is activated by a Brønsted acid such as C6F5CHTf2 and used for Diels-Alder reactions of mixtures of monosubstituted cyclopentadienes with ethyl acrylate. In these reactions, only 2-substituted cyclopentadienes are selectively reacted and afford endo-adducts with high regio- and enantio-selectivities. Subsequent Diels-Alder reactions with unreacted 1-substituted cyclopentadienes are achieved by using more highly reactive dienophiles and the corresponding endo-adducts are obtained regio- and enantio-selectively. This reaction is the first report to develop regio- and enantio-selective Diels-Alder reactions.