Maximum quantity allowed is 999
Please select the quantity
Ester-amide Exchange Reactions of Vinyl Esters Using N-Heterocyclic Carbene (NHC) Catalysts
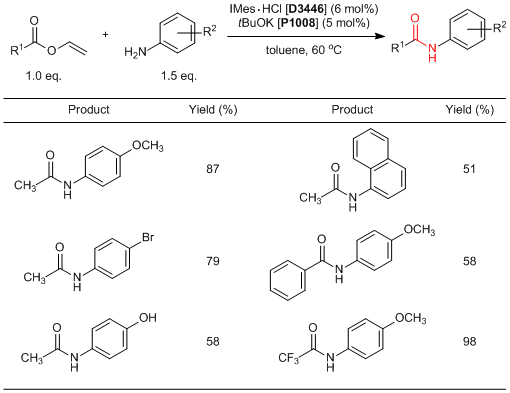
Du et al. have reported an amidation of vinyl esters using N-heterocyclic carbenes (NHC) as a catalyst. According to their results, the ester-amide exchange reactions of vinyl esters with primary aromatic amines successfully proceed in good yields by using NHC catalysts, which are prepared by the treatment of NHC precursors such as IMes·HCl and IPr·HCl imidazolium salts with a base (IMes and IPr are ready to be prepared, respectively). NMR experiments indicate that this reaction proceeds through two reaction mechanisms;" the NHC catalyst adds to a carbonyl group of vinyl esters to activate and act as a Brønsted base to activate an N-H bond of aromatic amines. Generally, amidation reactions require excess amounts of condensing agents and harsh reaction conditions such as heating under reflux. On the other hand, this amidation reaction proceeds easily under mild conditions, so that it is expected to be used in the future.
References
- N-Heterocyclic carbene-catalysed amidation of vinyl esters with aromatic amines