Maximum quantity allowed is 999
Please select the quantity
CAS RN: 719-98-2 | Product Number: T3143
N-(Trifluoromethylthio)phthalimide

Purity: >98.0%(GC)
Synonyms:
Product Documents:
| Size | Unit Price | Same Day | 2-3 Business Days |
|---|---|---|---|
| 1G |
$184.00
|
Contact Us | 7 |
| 5G |
$640.00
|
19 | Contact Us |
* Please contact our distributors or
TCI
to order our products. The above prices do not include freight cost, customs, and other charges to the destination.
* The storage conditions are subject to change without notice.
| Product Number | T3143 |
| Purity / Analysis Method | >98.0%(GC) |
| Molecular Formula / Molecular Weight | C__9H__4F__3NO__2S = 247.19 |
| Physical State (20 deg.C) | Solid |
| Storage Temperature | Room Temperature (Recommended in a cool and dark place, <15°C) |
| Store Under Inert Gas | Store under inert gas |
| Condition to Avoid | Air Sensitive |
| Packaging and Container | 1G-Glass Bottle with Plastic Insert (View image) |
| CAS RN | 719-98-2 |
| Reaxys Registry Number | 1464619 |
| PubChem Substance ID | 253662481 |
Specifications
| Appearance | White to Almost white powder to crystal |
| Purity(GC) | min. 98.0 % |
| Melting point | 111.0 to 115.0 °C |
Properties (reference)
| Melting Point | 113 °C |
GHS
| Pictogram |

|
| Signal Word | Warning |
| Hazard Statements | H315 : Causes skin irritation. H319 : Causes serious eye irritation. |
| Precautionary Statements | P264 : Wash skin thoroughly after handling. P280 : Wear protective gloves/ eye protection/ face protection. P302 + P352 : IF ON SKIN: Wash with plenty of water. P337 + P313 : If eye irritation persists: Get medical advice/ attention. P305 + P351 + P338 : IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. P362 + P364 : Take off contaminated clothing and wash it before reuse. P332 + P313 : If skin irritation occurs: Get medical advice/ attention. |
Related Laws:
Transport Information:
| H.S.code* | 2930.90-900 |
Application
Catalytic Trifluoromethylthiolation Using N-(Trifluoromethylthio)phthalimide
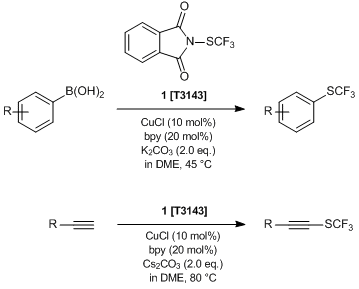
Typical Procedure (Reaction of 1 with boronic acids)3):
CuCl (4.0 mg, 0.04 mmol, 10 mol%), dry boronic acid (0.4 mmol, 1.0 eq.), dry K2CO3 (111 mg, 0.8 mmol, 2.0 eq.), 2,2’-bipyridine (12.5 mg, 0.08 mmol, 20 mol%) and N-(trifluoromethylthio)phthalimide (103.8 mg, 0.42 mmol, 1.05 eq.) are placed into an oven-dried Schlenk tube equipped with a stirring bar. The tube is sealed with a rubber stopper, evacuated and back-filled with dry argon (three times). Dry, freshly distilled and degassed DME is added by a syringe and the reaction mixture is stirred at 45 °C for 18 h. The reaction mixture is cooled to room temperature, diluted with diethyl ether (5 mL), and filtered through a short plug of silica, eluting with additional diethyl ether (50 mL). The filtrate is concentrated; pentane (10 mL) is added and concentrated again for removing residual DME. The resulting residue is purified by chromatography (pentane―diethyl ether) to provide the desired product.
CuCl (4.0 mg, 0.04 mmol, 10 mol%), dry boronic acid (0.4 mmol, 1.0 eq.), dry K2CO3 (111 mg, 0.8 mmol, 2.0 eq.), 2,2’-bipyridine (12.5 mg, 0.08 mmol, 20 mol%) and N-(trifluoromethylthio)phthalimide (103.8 mg, 0.42 mmol, 1.05 eq.) are placed into an oven-dried Schlenk tube equipped with a stirring bar. The tube is sealed with a rubber stopper, evacuated and back-filled with dry argon (three times). Dry, freshly distilled and degassed DME is added by a syringe and the reaction mixture is stirred at 45 °C for 18 h. The reaction mixture is cooled to room temperature, diluted with diethyl ether (5 mL), and filtered through a short plug of silica, eluting with additional diethyl ether (50 mL). The filtrate is concentrated; pentane (10 mL) is added and concentrated again for removing residual DME. The resulting residue is purified by chromatography (pentane―diethyl ether) to provide the desired product.
References
- 1)Trifluoromethylsulfenylation of masked carbonyl compounds
- 2)N-Trifluoromethylthiophthalimide: a stable electrophilic SCF3-reagent and its application in the catalytic asymmetric trifluoromethylsulfenylation
- 3)Direct catalytic trifluoromethylthiolation of boronic acids and alkynes employing electrophilic shelf-stable N-(trifluoromethylthio)phthalimide
PubMed Literature
Articles/Brochures
TCIMAIL
[Research Articles] Catalytic Trifluoromethylthiolation Using N-(Trifluoromethylthio)phthalimideProduct Documents (Note: Some products will not have analytical charts available.)
Safety Data Sheet (SDS)
Please select Language.
The requested SDS is not available.
Please Contact Us for more information.
Specifications
C of A & Other Certificates
Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
Sample C of A
This is a sample C of A and may not represent a recently manufactured lot of the product.
A sample C of A for this product is not available at this time.
Analytical Charts
Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
The requested analytical chart is not available. Sorry for the inconvenience.



