Maximum quantity allowed is 999
Please select the quantity
CAS RN: 144026-79-9 | Product Number: T1663
Scandium(III) Trifluoromethanesulfonate

Purity: >98.0%(T)
Synonyms:
- Scandium(III) Triflate
- Trifluoromethanesulfonic Acid Scandium(III) Salt
Product Documents:
* Please contact our distributors or
TCI
to order our products. The above prices do not include freight cost, customs, and other charges to the destination.
* The storage conditions are subject to change without notice.
| Product Number | T1663 |
| Purity / Analysis Method | >98.0%(T) |
| Molecular Formula / Molecular Weight | C__3F__9O__9S__3Sc = 492.15 |
| Physical State (20 deg.C) | Solid |
| Storage Temperature | Room Temperature (Recommended in a cool and dark place, <15°C) |
| Store Under Inert Gas | Store under inert gas |
| Condition to Avoid | Hygroscopic |
| Packaging and Container | 1G-Glass Bottle with Plastic Insert (View image) |
| CAS RN | 144026-79-9 |
| Reaxys Registry Number | 8510151 |
| PubChem Substance ID | 87577404 |
| MDL Number | MFCD00192433 |
Specifications
| Appearance | White to Almost white powder to crystal |
| Purity(Chelometric Titration) | min. 98.0 % |
Properties (reference)
| Solubility in water | Soluble |
GHS
| Pictogram |

|
| Signal Word | Warning |
| Hazard Statements | H315 : Causes skin irritation. H319 : Causes serious eye irritation. |
| Precautionary Statements | P264 : Wash skin thoroughly after handling. P280 : Wear protective gloves/ eye protection/ face protection. P302 + P352 : IF ON SKIN: Wash with plenty of water. P337 + P313 : If eye irritation persists: Get medical advice/ attention. P305 + P351 + P338 : IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. P362 + P364 : Take off contaminated clothing and wash it before reuse. P332 + P313 : If skin irritation occurs: Get medical advice/ attention. |
Related Laws:
Transport Information:
| H.S.code* | 2846.90-000 |
Application
Acetylation of Alcohols using Acetylating Reagents and Acid Catalysts
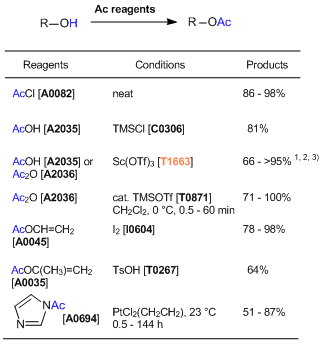
References
- 1)A Novel Method for the Preparation of Macrolides from ω-Hydroxycarboxylic Acids
- 2)An Efficient Esterification Reaction between Equimolar Amounts of Free Carboxylic Acids and Alcohols by the Combined Use of Octamethylcyclotetrasiloxane and a Catalytic Amount of Titanium(IV) Chloride Tris(trifluoromethanesulfonate)
- 3)Scandium Trifluoromethanesulfonate as an Extremely Active Lewis Acid Catalyst in Acylation of Alcohols with Acid Anhydrides and Mixed Anhydrides
- 4)Protection for the Hydroxyl Group, Including 1,2- and 1,3-Diols
- P. G. M. Wuts, in Greene’s Protective Groups in Organic Synthesis, 5th ed., ed. by P. G. M. Wuts, John Wiley & Sons, Inc., Hoboken, New Jersey, 2014, Chap. 2, 17.
Application
Stereoinversion of tertiary alcohols to tertiary-alkyl isonitriles

Typical Procedure (entry 1): A solution of (-)-α-bisabolol (22.2 mg, 0.1 mmol) in anhydrous dichloromethane (0.15 mL) is cooled to 0 °C and treated with anhydrous pyridine (31.6 mg, 0.4 mmol), followed by trifluoroacetic anhydride (42 mg, 0.2 mmol). The reaction mixture is stirred at 0 °C for 20 min and quenched with 1 N aqueous HCl. The resulting biphasic mixture is stirred vigorously at room temperature for 5 min and extracted twice with hexanes. The combined organic layers are washed with water followed by saturated aqueous NaHCO3, dried over anhydrous Na2SO4, and concentrated under reduced pressure. The resulting crude trifluoroacetate (32.0 mg, 0.1 mmol) is dissolved in TMSCN (0.1 mL), cooled to 0 °C, and treated drop-wise with a solution of anhydrous Sc(OTf)3 (1.5 mg, 0.003 mmol) in TMSCN (0.1 mL). The reaction mixture is left at 3 °C for 18 h and quenched with tetramethylethylenediamine (7.5 µl, 0.05 mmol). The resulting solution is concentrated under reduced pressure, and the residue is purified by flash column chromatography on silica gel (eluent: 35% dichloromethane in hexanes) to give 7-isocyano-7,8-dihydro-α-bisabolene (18.0 mg, 78% yield, dr 83:17) as a light yellow oil.
References
Application
Reusable Lewis Acid
References
- A novel reusable Lewis acid catalyst in aldol and Michael reactions
- A novel reusable catalyst in Diels-Alder reaction
- A chiral scandium catalyst for enantioselective Diels-Alder reactions
- Aqueous reactions with Lewis acid and organometalic reagent
PubMed Literature
Articles/Brochures
Product Documents (Note: Some products will not have analytical charts available.)
Safety Data Sheet (SDS)
Please select Language.
The requested SDS is not available.
Please Contact Us for more information.
Specifications
C of A & Other Certificates
Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
Sample C of A
This is a sample C of A and may not represent a recently manufactured lot of the product.
A sample C of A for this product is not available at this time.
Analytical Charts
Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
The requested analytical chart is not available. Sorry for the inconvenience.




