Maximum quantity allowed is 999
Please select the quantity
A Convenient Nucleophilic Iodinating Reagent
No.161(April 2014)
N,N-Dimethyl-N-(methylsulfanylmethylene)ammonium iodide (1) developed by Porter et al. is a novel nucleophilic iodinating reagent. It can convert primary and secondary alcohols to the corresponding iodo compounds. Though it is generally difficult to accomplish the iodination of allylic alcohols, 1 can replace them with the desired allyl iodides. In addition, primary alcohols react more rapidly than secondary alcohols, which allows selective iodination of primary hydroxy groups in primary-secondary diols. The similar iodinations using triphenylphosphine/iodinating reagents generate stoichiometric amounts of triphenylphosphine oxide, which often causes difficulty in product purification. In contrast, iodination using 1 has advantages such as easy work-up and product purification. Thus, 1 can be used as a convenient nucleophilic iodinating reagent.
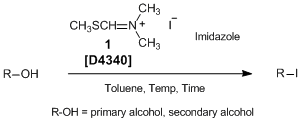
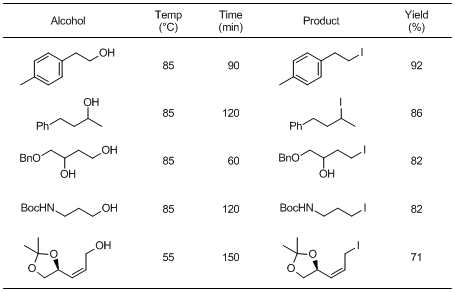
Typical Procedure: Iodination of 2-(4-methylphenyl)ethanol
A solution of 2-(4-methylphenyl)ethanol (102 mg, 0.75 mmol) in toluene (3.5 mL) is heated to 85 °C. 1 (260 mg, 1.12 mmol) and imidazole (25 mg, 0.37 mmol) are added and the mixture is stirred at 85 °C for 90 min. After cooling to room temperature, the reaction mixture is concentrated in vacuo to afford the crude product. Purification by flash chromatography (SiO2; petrol) affords 1-iodo-2-(4-methylphenyl)ethane as a colorless oil (170 mg, Y. 92%).
A solution of 2-(4-methylphenyl)ethanol (102 mg, 0.75 mmol) in toluene (3.5 mL) is heated to 85 °C. 1 (260 mg, 1.12 mmol) and imidazole (25 mg, 0.37 mmol) are added and the mixture is stirred at 85 °C for 90 min. After cooling to room temperature, the reaction mixture is concentrated in vacuo to afford the crude product. Purification by flash chromatography (SiO2; petrol) affords 1-iodo-2-(4-methylphenyl)ethane as a colorless oil (170 mg, Y. 92%).
References
- Selective conversion of alcohols into alkyl iodides using a thioiminium salt
The prices are subject to change without notice. Please confirm the newest price by our online catalog before placing an order.
In addition, sales products changes with areas. Please understand that a product is not available when the product details page is not displayed.
In addition, sales products changes with areas. Please understand that a product is not available when the product details page is not displayed.