Maximum quantity allowed is 999
Please select the quantity
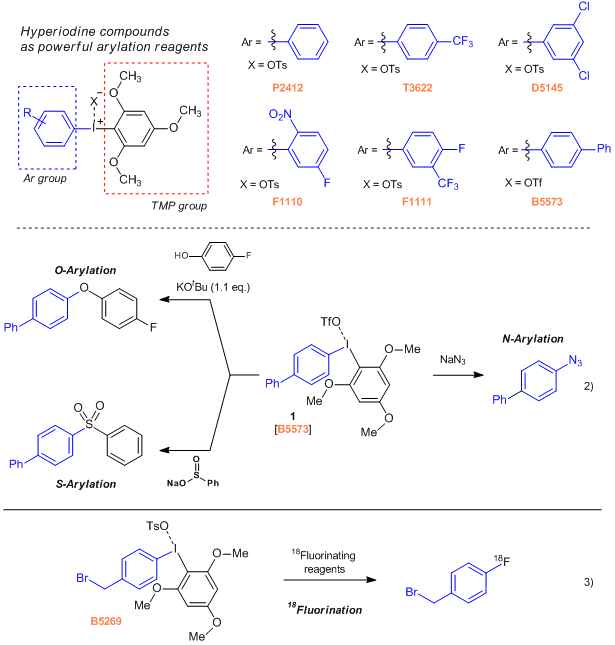
Asymmetric Diaryliodonium Tosylates Useful for Metal-free Arylations
Asymmetric diaryliodonium salts bearing 1,3,5-trimethoxyphenyl (TMP) and an aryl group, are applicable to multiple arylation reactions without using a transition metal complex.1) For example, the 4-biphenylyl derivative (1) can smoothly react with phenol, sodium benzenesulfinate, and sodium azide as O-, N- and S- nucleophiles to give the corresponding O-, N- and S-aryl products.2) Furthermore, reactions with 18fluorinating agents have been reported. This 18F labeling procedure can be applied to the synthesis of molecular probe contrast agents for PET (Positron Emission Tomography) scan diagnostics.3)
Related Products
- P2412
- Phenyl(2,4,6-trimethoxyphenyl)iodonium p-Toluenesulfonate
- T3622
- [(4-Trifluoromethyl)phenyl](2,4,6-trimethoxyphenyl)iodonium p-Toluenesulfonate
- D5145
- (3,5-Dichlorophenyl)(2,4,6-trimethoxyphenyl)iodonium p-Toluenesulfonate
- F1110
- (5-Fluoro-2-nitrophenyl)(2,4,6-trimethoxyphenyl)iodonium p-Toluenesulfonate
- F1111
- [4-Fluoro-3-(trifluoromethyl)phenyl](2,4,6-trimethoxyphenyl)iodonium p-Toluenesulfonate
- B5573
- 4-Biphenylyl(2,4,6-trimethoxyphenyl)iodonium Trifluoromethanesulfonate
- B5269
- [4-(Bromomethyl)phenyl](2,4,6-trimethoxyphenyl)iodonium p-Toluenesulfonate
References
- 1)Aryl(2,4,6-trimethoxyphenyl)iodonium Salts as Reagents for Metal-Free Arylation of Carbon and Heteroatom Nucleophiles
- 2)Unsymmetrical Aryl(2,4,6-trimethoxyphenyl)iodonium Salts: One-Pot Synthesis, Scope, Stability, and Synthetic Studies
- 3)Single-step syntheses of no-carrier-added functionalized [18F]fluoroarenes as labeling synthons from diaryliodonium salts