Maximum quantity allowed is 999
Please select the quantity
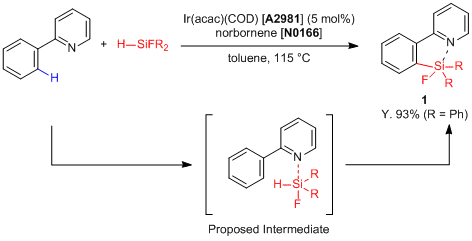
ortho-Selective C-H Silylation of Aryl Compounds Using an Iridium Catalyst
Kuninobu, Kanai et al. have reported an iridium-catalyzed regioselective silylation of aryl compounds.1) According to their results, in the presence of Ir(acac)(COD) catalyst, benzene derivatives possessing the directing group like a 2-pyridyl group successfully react with fluorinated hydrosilanes to proceed with the ortho-selective C-H silylation, and the desired silafluorene equivalents 1 are given. This reaction indicates that Lewis acidity of a silicon atom is important to promote the silylation because the reaction is not promoted when using a non-fluorinated hydrosilane, and this silylation proceeds with an interaction between fluorinated hydrosilanes and the Lewis basic 2-pyridyl group. Silafluorene equivalents show strong fluorescence and high quantum yield,2) so that this reaction is expected to be applied for development of organic materials.
References
- 1) Iridium-Catalyzed ortho-Selective C−H Silylation of Aromatic Compounds Directed toward the Synthesis of π‑Conjugated Molecules with Lewis Acid−Base Interaction
- 2) Palladium-Catalyzed C-H Fluorosilylation of 2-Phenylpyridines: Synthesis of Silafluorene Equivalents