Maximum quantity allowed is 999
Please select the quantity
CAS RN: 64443-05-6 | Product Number: T2665
Tetrakis(acetonitrile)copper(I) Hexafluorophosphate
Purity: >97.0%(T)
Synonyms:
Product Documents:
| Size | Unit Price | Shanghai | Tianjin | Japan* |
|---|---|---|---|---|
| 5G |
¥770.00
|
≥80 | 6 | ≥100 |
* For order or inquiry, please contact
Our Authorized Distributors.
*TCI frequently reviews storage conditions to optimize them. Please note that the latest information on the storage temperature for the products is described on our website.
* To send your quote request for bulk quantities, please click on the "Request Bulk Quote" button. Please note that we cannot offer bulk quantities for some products.
| Product Number | T2665 |
| Purity / Analysis Method | >97.0%(T) |
| Molecular Formula / Molecular Weight | C__8H__1__2CuF__6N__4P = 372.72 |
| Physical State (20 deg.C) | Solid |
| Storage Temperature | Frozen (<0°C) |
| Store Under Inert Gas | Store under inert gas |
| Condition to Avoid | Air Sensitive,Moisture Sensitive,Heat Sensitive |
| CAS RN | 64443-05-6 |
| Reaxys Registry Number | 14262634 |
| PubChem Substance ID | 87561580 |
| MDL Number | MFCD00064810 |
Specifications
| Appearance | White to Almost white powder to crystal |
| Purity(Potassium permanganate method) | min. 97.0 % |
| NMR | confirm to structure |
Properties (reference)
| Melting Point | 160 °C(dec.) |
GHS
| Pictogram |

|
| Signal Word | Warning |
| Hazard Statements | H315 : Causes skin irritation. H319 : Causes serious eye irritation. |
| Precautionary Statements | P264 : Wash skin thoroughly after handling. P280 : Wear protective gloves/ eye protection/ face protection. P302 + P352 : IF ON SKIN: Wash with plenty of water. P337 + P313 : If eye irritation persists: Get medical advice/ attention. P305 + P351 + P338 : IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. P362 + P364 : Take off contaminated clothing and wash it before reuse. P332 + P313 : If skin irritation occurs: Get medical advice/ attention. |
Related Laws:
Transport Information:
| Customs Control Conditions (Q) |
Application
Perfluoroalkylation using Perfluoro Acid Anhydrides

Reference
- Perfluoroalkylation of Unactivated Alkenes with Acid Anhydrides as the Perfluoroalkyl Source
Application
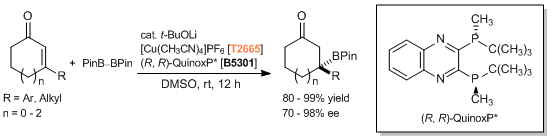
Copper Catalyzed Asymmetric Conjugate Addition
References
- Catalytic Asymmetric Synthesis of Chiral Tertiary Organoboronic Esters through Conjugate Boration of β-Substituted Cyclic Enones
- Searching for Practically Useful P-Chirogenic Phosphine Ligands
Application
Catalytic Asymmetric Vinylogous Conjugate Addition of Unsaturated Butyrolactones to α,β-Unsaturated Thioamides
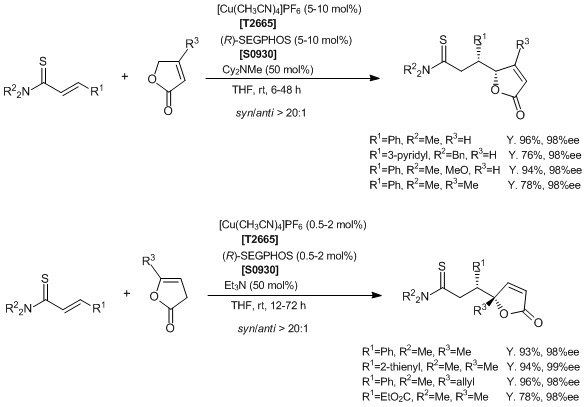
Reference
Application
Copper-catalyzed C―H Amidation of Unactivated Arenes

Typical Procedure:
To a stirred mixture of [Cu(CH3CN)4]PF6 (0.010 g, 0.026 mmol) and neocuproine (0.005 g, 0.024 mmol) in the arene (ca. 1 mL) is added N-tosyloxy-2,2,2-trichloroethylcarbamate (0.050 g, 0.138 mmol) and the mixture is allowed to stir at 140 °C for 8 h. The reaction mixture is cooled to room temperature, filtered through a pad of silica, washed with CH2Cl2, and the filtrate is concentrated under vacuum. The residue is purified by silica chromatography using 5–15% Et2O in hexanes to obtain the regioisomeric mixture of aminated arenes. The isomer ratio is determined by NMR using 1,3,5-trimethoxybenzene as a reference.
To a stirred mixture of [Cu(CH3CN)4]PF6 (0.010 g, 0.026 mmol) and neocuproine (0.005 g, 0.024 mmol) in the arene (ca. 1 mL) is added N-tosyloxy-2,2,2-trichloroethylcarbamate (0.050 g, 0.138 mmol) and the mixture is allowed to stir at 140 °C for 8 h. The reaction mixture is cooled to room temperature, filtered through a pad of silica, washed with CH2Cl2, and the filtrate is concentrated under vacuum. The residue is purified by silica chromatography using 5–15% Et2O in hexanes to obtain the regioisomeric mixture of aminated arenes. The isomer ratio is determined by NMR using 1,3,5-trimethoxybenzene as a reference.
References
PubMed Literature
Articles/Brochures
TCIMAIL
[Research Articles] Perfluoroalkylation using Perfluoro Acid Anhydrides[Research Articles] Catalytic Asymmetric Vinylogous Conjugate Addition of Unsaturated Butyrolactones to α,β-Unsaturated Thioamides
[Research Articles] Copper-catalyzed C—H Amidation of Unactivated Arenes
Product Documents (Note: Some products will not have analytical charts available.)
Safety Data Sheet (SDS)
Please select Language.
The requested SDS is not available.
Please Contact Us for more information.
Specifications
C of A & Other Certificates
Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
Sample C of A
This is a sample C of A and may not represent a recently manufactured lot of the product.
A sample C of A for this product is not available at this time.
Analytical Charts
Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
The requested analytical chart is not available. Sorry for the inconvenience.



