Maximum quantity allowed is 999
CAS RN: 19350-66-4 | Product Number: B6316
3,5-Bis(ethoxycarbonyl)-2,6-dimethyl-1,4-dihydropyridine-4-carboxylic Acid

Purity: >95.0%(T)(HPLC)
- 2,6-Dimethyl-1,4-dihydro-pyridine-3,4,5-tricarboxylic Acid 3,5-Diethyl Ester
- 3,5-Bis(ethoxycarbonyl)-2,6-dimethyl-1,4-dihydro-4-pyridinecarboxylic Acid
| Size | Unit Price | Shanghai | Tianjin | Japan* |
|---|---|---|---|---|
| 1G |
¥690.00
|
Contact Us | Contact Us | 14 |
| 10G |
¥3,990.00
|
Contact Us | Contact Us | 8 |
* For order or inquiry, please contact
Our Authorized Distributors.
*TCI frequently reviews storage conditions to optimize them. Please note that the latest information on the storage temperature for the products is described on our website.
* To send your quote request for bulk quantities, please click on the "Request Bulk Quote" button. Please note that we cannot offer bulk quantities for some products.
| Product Number | B6316 |
| Purity / Analysis Method | >95.0%(T)(HPLC) |
| Molecular Formula / Molecular Weight | C__1__4H__1__9NO__6 = 297.31 |
| Physical State (20 deg.C) | Solid |
| Storage Temperature | Room Temperature (Recommended in a cool and dark place, <15°C) |
| Store Under Inert Gas | Store under inert gas |
| Condition to Avoid | Air Sensitive |
| CAS RN | 19350-66-4 |
| Reaxys Registry Number | 489397 |
| PubChem Substance ID | 468591034 |
| MDL Number | MFCD00417306 |
| Appearance | White to Light yellow powder to crystal |
| Purity(HPLC) | min. 95.0 area% |
| Purity(Neutralization titration) | min. 95.0 % |
| NMR | confirm to structure |
| Melting Point | 220 °C |
| Pictogram |

|
| Signal Word | Warning |
| Hazard Statements | H315 : Causes skin irritation. H319 : Causes serious eye irritation. |
| Precautionary Statements | P264 : Wash skin thoroughly after handling. P280 : Wear protective gloves/ eye protection/ face protection. P302 + P352 : IF ON SKIN: Wash with plenty of water. P337 + P313 : If eye irritation persists: Get medical advice/ attention. P305 + P351 + P338 : IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. P362 + P364 : Take off contaminated clothing and wash it before reuse. P332 + P313 : If skin irritation occurs: Get medical advice/ attention. |
| Customs Control Conditions (Q) |
-
Used Chemicals
-
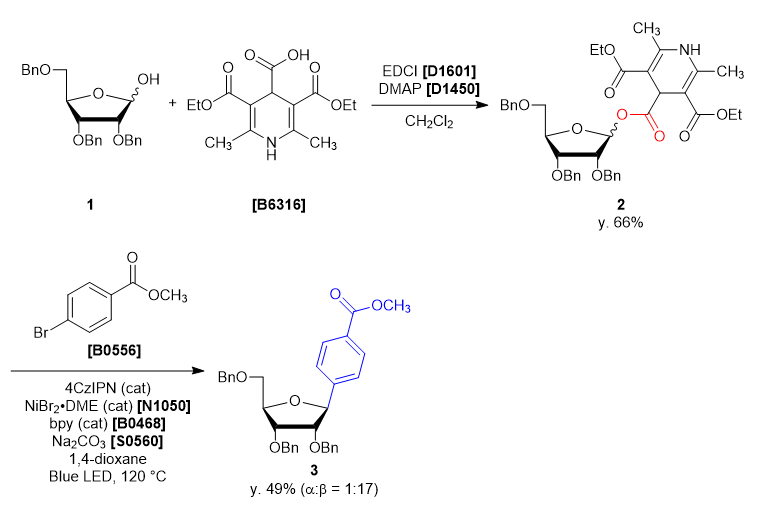
- 3,5-Bis(ethoxycarbonyl)-2,6-dimethyl-1,4-dihydropyridine-4-carboxylic Acid [B6316]
- 2,3,5-Tri-O-benzyl-α/β-D-ribofuranose (1)
- 4-Dimethylaminopyridine (= DMAP) [D1450]
- 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide Hydrochloride (= EDCI) [D1601]
- Dichloromethane
- Sodium Carbonate [S0560]
- 1,2,3,5-Tetrakis(carbazol-9-yl)-4,6-dicyanobenzene (= 4CzIPN)
- Methyl 4-Bromobenzoate [B0556]
- 2,2'-Bipyridyl (= bpy) [B0468]
- Nickel(II) Bromide Ethylene Glycol Dimethyl Ether Complex (= NiBr2・DME) [N1050]
- 1,4-Dioxane
-
Procedure
-
A four-neck round bottom flask was charged with 2,3,5-tri-O-benzyl-α/β-D-ribofuranose (1) (2.165 g, 5.15 mmol, 1 eq) and dichloromethane (26 mL). The solution was cooled under 5 ˚C, then DMAP (0.063 g, 0.51 mmol, 0.1 eq), EDCI (1.38 g, 7.21 mmol, 1.4 eq), 3,5-bis(ethoxycarbonyl)-2,6-dimethyl-1,4-dihydropyridine-4-carboxylic acid (1.684 g, 5.66 mmol, 1.1 eq) was added. The reaction mixture was allowed to warm to ambient temperature and stirred for 46 h. After the reaction, the reaction mixture was quenched with ion-exchanged water (35 mL). The solution was transferred into a separatory funnel, and the aqueous layer was extracted with dichloromethane (10 mL, twice). The combined organic layers were washed with brine, dried over sodium sulfate (10 g) for about 30 minutes and then filtered. The solvent was removed in vacuo, giving crude as a blown oil (3.91 g). The crude was purified by silica gel column chromatography (hexane:ethyl acetate = 1:1, Rf = 0.45) to give compound 2 as a light yellow oil (2.99 g, y. 66%).
30 mL sealed vessel was charged with 4CzIPN (0.0158 g, 0.020 mmol, 0.1 eq), methyl 4-bromobenzoate (0.0858 g, 0.399 mmol, 2.0 eq), sodium carbonate (0.0443 g, 0.418 mmol, 2.1 eq), 2,2’-bipyridine (0.0086 g, 0.055 mmol, 0.28 eq) at rt under N2. A solution of NiBr2・DME (0.012 g, 0.040 mmol, 0.20 eq) in 1,4-dioxane (5 mL) and the solution of 1 (0.14 g, 0.20 mmol, 1.0 eq) in 1,4-dioxane (5 mL) were added to the sealed vessel. The mixture was placed in a preheated oil bath whose temperature was set to 120 ˚C, and placed at a distance of 2-3 cm from Blue LED lamp. After irradiation for 46 h, the reaction mixture was cooled to room temperature and filtered through Celite pad (1 cm). The solvent was removed in vacuo and the crude was given as a yellow oil (0.23 g). The crude was purified by silica gel column chromatography (hexane:ethyl acetate = 10:2, Rf = 0.30) to give compound 3 as a colorless oil (0.053 g, y. 49%). -
-
Experimenter’s Comments
-
- NiBr2・DME was weighed in a nitrogen-filled glove box and dissolved in 1,4-dioxane completely using a sonication.
- 1,4-Dioxane was degassed with nitrogen for 30 min before use.
- Irradiation of visible light was performed with Kessil A160WE Tuna Blue 40W x 2.
- The reaction mixture was monitored by 1H NMR and UPLC.
- The α/β selectivity of 3 was 1:17.
-
Analytical Data
-
Compound 3
1H NMR (270 MHz, CDCl3); δ 7.96 (d, J = 8.2 Hz, 2H), 7.46 (d, J = 8.2 Hz, 2H), 7.40-7.30 (m, 11H), 7.26-7.23 (m, 2H), 7.18-7.15 (m, 2H), 5.05 (d, J = 6.9 Hz, 1H), 4.71-4.44 (m, 6H), 4.39-4.34 (m, 1H), 4.01 (dd, J = 5.3, 3.3 Hz, 1H), 3.92 (s, 3H), 3.77 (dd, J = 6.9, 5.3 Hz, 1H), 3.65 (qd, J = 10.6, 3.9 Hz, 2H).
-
Lead Reference
-
- Diastereoselective Synthesis of Aryl C-Glycosides from Glycosyl Esters via C-O Bond Homolysis
-
Other Reference
-
- Highly stereoselective synthesis of aryl/heteroaryl-C-nucleosides via the merger of photoredox and nickel catalysis
Articles/Brochures
Safety Data Sheet (SDS)
The requested SDS is not available.
Please Contact Us for more information.
Specifications
C of A & Other Certificates
Sample C of A
A sample C of A for this product is not available at this time.
Analytical Charts
The requested analytical chart is not available. Sorry for the inconvenience.



