Maximum quantity allowed is 999
CAS RN: 119752-83-9 | Product Number: B3960
Bis(sulfur Dioxide)-1,4-diazabicyclo[2.2.2]octane Adduct
![Bis(sulfur Dioxide)-1,4-diazabicyclo[2.2.2]octane Adduct No-Image](/medias/B3960.jpg?context=bWFzdGVyfHJvb3R8NTkxMTF8aW1hZ2UvanBlZ3xhREZrTDJnMFlTODRPVEl3TVRnd016STJORE13TDBJek9UWXdMbXB3Wnd8MDlhZDVmMDUzNTU2OTI4OGU3MzVjYWFlOWYyYzcyNGYzYzI3NzM0NjQ2ODBhNDI3MzYzOWM3ZDRiZmEzZjRhNw)
Purity: >94.0%(T)
- DABCO-Bis(sulfur Dioxide)
- DABSO
| Size | Unit Price | Shanghai | Tianjin | Japan* |
|---|---|---|---|---|
| 1G |
¥380.00
|
26 | 2 | ≥40 |
| 5G |
¥1,180.00
|
≥40 | 6 | ≥80 |
* For order or inquiry, please contact
Our Authorized Distributors.
*TCI frequently reviews storage conditions to optimize them. Please note that the latest information on the storage temperature for the products is described on our website.
* To send your quote request for bulk quantities, please click on the "Request Bulk Quote" button. Please note that we cannot offer bulk quantities for some products.
| Product Number | B3960 |
| Purity / Analysis Method | >94.0%(T) |
| Molecular Formula / Molecular Weight | C__6H__1__2N__2O__4S__2 = 240.29 |
| Physical State (20 deg.C) | Solid |
| Storage Temperature | Refrigerated (0-10°C) |
| Store Under Inert Gas | Store under inert gas |
| Condition to Avoid | Air Sensitive,Heat Sensitive |
| Packaging and Container | 1G-Glass Bottle with Plastic Insert (View image) |
| CAS RN | 119752-83-9 |
| Reaxys Registry Number | 21034099 |
| PubChem Substance ID | 354335121 |
| Appearance | White to Almost white powder to crystal |
| Purity(Iodometric back titration) | min. 94.0 % |
| Melting Point | 180 °C(dec.) |
| Solubility in water | Soluble |
| Pictogram |

|
| Signal Word | Warning |
| Hazard Statements | H315 : Causes skin irritation. H319 : Causes serious eye irritation. |
| Precautionary Statements | P264 : Wash skin thoroughly after handling. P280 : Wear protective gloves/ eye protection/ face protection. P302 + P352 : IF ON SKIN: Wash with plenty of water. P337 + P313 : If eye irritation persists: Get medical advice/ attention. P305 + P351 + P338 : IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. P362 + P364 : Take off contaminated clothing and wash it before reuse. P332 + P313 : If skin irritation occurs: Get medical advice/ attention. |
| Customs Control Conditions (Q) |

-
Used Chemicals
-
- 4-Biphenylboronic Acid (contains varying amounts of Anhydride) [B2294]
- Nickel(II) Bromide Ethylene Glycol Dimethyl Ether Complex (=NiBr2(glyme)) [N1050]
- 3,4,7,8-Tetramethyl-1,10-phenanthroline (= tmphen) [T0847]
- Bis(sulfur Dioxide)-1,4-diazabicyclo[2.2.2]octane Adduct (= DABSO) [B3960]
- Lithium tert-Butoxide (= LiOtBu)
- tert-Butyl Bromoacetate [B1473]
- 1,3-Dimethyl-2-imidazolidinone (= DMI) [D1477]
-
Procedure
-
Under nitrogen atmosphere, a degassed DMI (20 mL) solution of 4-biphenylboronic acid (792 mg, 4.00 mmol), DABSO (577 mg, 2.40 mmol), tmphen (95 mg, 0.40 mmol), lithium tert-butoxide (320 mg, 4.00 mmol) and NiBr2(glyme) (123 mg, 0.40 mmol) was stirred at 100 °C for 14 hours. The mixture was cooled to room temperature and tert-butyl bromoacetate (1.17 mL, 8.00 mmol) was added and the reaction mixture stirred for 1 hour. Water (20 mL) was poured into the reaction mixture and the mixture was extracted with diethyl ether (50 mL x 3). The combined organic phase was washed with water (50 mL) and brine (40 mL), dried over sodium sulfate and filtered. The solvent was removed under reduced pressure and the residue was purified by medium pressure preparative chromatography (ethyl acetate:hexane = 5:95 - 15:85 on silica gel) to give compound 1 as a white solid (1.01 g, 76%).
-
Experimenter’s Comments
-
NiBr2(glyme) and LiOtBu were weighed into a sample tube in a glove box because these reagents are moisture sensitive. If these were used in air, it should be weighted quickly and used up the reagents as soon as possible after opening.
Since the ligand of NiBr2(glyme) may be desorbed under reduced pressure, depressurization operations is desirable to be finished as soon as possible.
The reaction mixture was monitored by TLC (ethyl acetate:hexane = 2:1, Rf = 0.38) and UPLC-MS.
-
Analytical Data
-
Compound 1
1H NMR (400 MHz, CDCl3); δ 8.00 (d, 2H, J = 8.9 Hz), 7.78 (m, 2H), 7.58–7.66 (m, 2H), 7.42-7.54 (m, 3H), 4.08 (s, 2H), 1.58 (s, 2H), 1.39 (s, 9H).
-
Lead Reference
-
- Nickel(II)-Catalyzed Synthesis of Sulfinates from Aryl and Heteroaryl Boronic Acids and the Sulfur Dioxide Surrogate DABSO

-
Used Chemicals
-
Procedure
-
To a solution of 4-bromobiphenyl (1.17 g, 5.00 mmol), DABSO (721 mg, 3.00 mmol), and PdCl2(Amphos)2 (177 mg, 0.25 mmol) in anhydrous isopropyl alcohol (20 mL) was added triethylamine (2.1 mL, 15 mmol) at room temperature and the mixture was stirred at 75 °C for 23 hours. The suspension was cooled at room temperature and NFSI (2.36 g, 7.5 mmol) was added and the reaction mixture stirred for 3 hours until completion. The solvent was removed under reduced pressure. The residue was diluted with ethyl acetate (20 mL) and filtrated through celite pad. The filtrate was washed with saturated aqueous Na2S2O3 solution (20 mL) and brine (20 mL), dried over sodium sulfate and filtered. The solvent was removed under reduced pressure and the residue was purified by medium pressure preparative chromatography (ethyl acetate:hexane = 2:98 - 5:95 on silica gel) to give compound 1 as a white solid (928 mg, 79%).
-
Experimenter’s Comments
-
The reaction mixture was monitored by TLC (ethyl acetate:hexane = 1:9, Rf = 0.57) and UPLC-MS.
-
Analytical Data
-
Compound 1
1H NMR (400 MHz, CDCl3); δ 8.12–8.04 (m, 2H), 7.86–7.80 (m, 2H), 7.67–7.58 (m, 2H), 7.44-7.55 (m, 3H).
-
Lead Reference
-
- One-pot palladium-catalyzed synthesis of sulfonyl fluorides from aryl bromides

Reference
- Copper(I)-catalyzed Sulfonylative Suzuki?Miyaura Cross-coupling
Reference
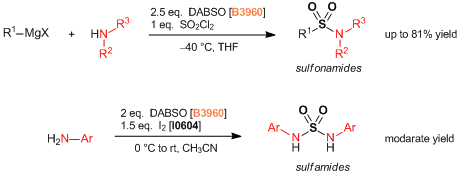
- DABCO- Bis (sulfur dioxide), DABSO, as a Convenient Source of Sulfur Dioxide for Organic Synthesis: Utility in Sulfonamide and Sulfamide Preparations
Reference
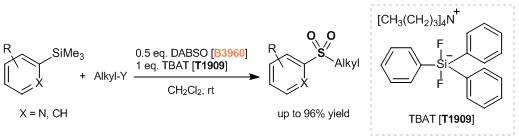
- Synthesis of Aromatic Sulfones from SO2 and Organosilanes Under Metal-free Conditions
Articles/Brochures
[Product Highlights] DABSO as a SO2 Source Usable for Direct Sulfonylative-Suzuki Coupling of Aryl Boronic Acids
[TCI Practical Example] One-Pot Palladium-Catalyzed Fluorosulfonylation of an Aryl Bromide Using DABSO and NFSI
Safety Data Sheet (SDS)
The requested SDS is not available.
Please Contact Us for more information.
Specifications
C of A & Other Certificates
Sample C of A
A sample C of A for this product is not available at this time.
Analytical Charts
The requested analytical chart is not available. Sorry for the inconvenience.

![Bis(sulfur Dioxide)-1,4-diazabicyclo[2.2.2]octane Adduct Bis(sulfur Dioxide)-1,4-diazabicyclo[2.2.2]octane Adduct](/_ui/responsive/theme-tci/images/missing_product_zoom.jpg)

