Published TCIMAIL newest issue No.197
Maximum quantity allowed is 999
Please select the quantity
CAS RN: 109-63-7 | Product Number: B0527
Boron Trifluoride - Ethyl Ether Complex
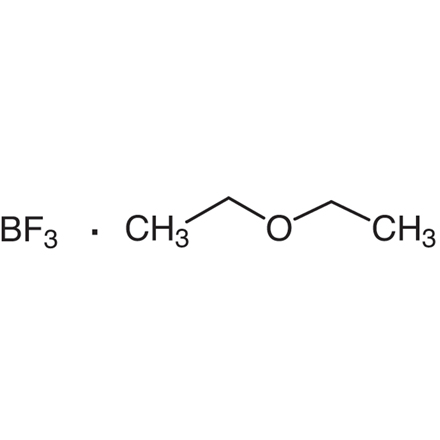
Purity: >98.0%(W)
Synonyms:
- Boron Trifluoride - Diethyl Ether Complex
Product Documents:
| Size | Unit Price | Belgium | Japan* | Quantity |
|---|---|---|---|---|
| 25ML |
17,00 €
|
29 | ≥100 |
|
| 100ML |
21,00 €
|
≥80 | ≥80 |
|
| 500ML |
61,00 €
|
7 | ≥100 |
|
*Stock available in Belgium will be delivered in 1 to 3 days
*Stock available in Japan will be delivered in 1 to 2 weeks (excludes regulated items and dry ice shipments).
Supplemental Product Information:
Both 100mL and 500mL bottles of this product are attached with a DualSeal (Septum-type Bottle Cap).
For details, please refer to the page "DualSeal (Septum-type Bottle Cap)".
| Product Number | B0527 |
| Purity / Analysis Method | >98.0%(W) |
| Molecular Formula / Molecular Weight | BF__3·C__4H__1__0O = 141.93 |
| Physical State (20 deg.C) | Liquid |
| Storage Temperature | Room Temperature (Recommended in a cool and dark place, <15°C) |
| Store Under Inert Gas | Store under inert gas |
| Condition to Avoid | Air Sensitive,Moisture Sensitive |
| Packaging and Container | 100ML-DualSeal Bottle (View image), 25ML-DualSeal Bottle (View image), 500ML-DualSeal Bottle (View image) |
| CAS RN | 109-63-7 |
| Reaxys Registry Number | 3909607 |
| PubChem Substance ID | 87563549 |
| Merck Index (14) | 1350 |
| MDL Number | MFCD00013194 |
Specifications
| Appearance | Colorless to Yellow to Orange clear liquid |
| Purity(Gravimetric) | 98.0 to 102.0 % |
| NMR | confirm to structure |
Properties (reference)
| Melting Point | -60 °C |
| Boiling Point | 124 °C |
| Specific Gravity (20/20) | 1.13 |
| Refractive Index | 1.34 |
| Solubility (soluble in) | Alcohol, Ether |
GHS
| Pictogram |



|
| Signal Word | Danger |
| Hazard Statements | H302 + H312 : Harmful if swallowed or in contact with skin. H314 : Causes severe skin burns and eye damage. H226 : Flammable liquid and vapour. |
| Precautionary Statements | P210 : Keep away from heat, hot surfaces, sparks, open flames and other ignition sources. No smoking. P280 : Wear protective gloves/ protective clothing/ eye protection/ face protection/ hearing protection. P370 + P378 : In case of fire: Use dry sand, dry chemical or alcohol-resistant foam to extinguish. P303 + P361 + P353 : IF ON SKIN (or hair): Take off immediately all contaminated clothing. Rinse skin with water. P304 + P340 + P310 : IF INHALED: Remove person to fresh air and keep comfortable for breathing. Immediately call a POISON CENTER/doctor. P305 + P351 + P338 + P310 : IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. Immediately call a POISON CENTER/doctor. |
Related Laws:
| EC Number | 203-689-8 |
Transport Information:
| UN Number | UN2604 |
| Class | 8 / 3 |
| Packing Group | I |
| HS Number | 3822190090 |
Application
t-Bu protection of Alcohols using Isobutylene

References
- Protection for the Hydroxyl Group, Including 1,2- and 1,3-Diols
- P. G. M. Wuts, in Greene’s Protective Groups in Organic Synthesis, 5th ed., ed. by P. G. M. Wuts, John Wiley & Sons, Inc., Hoboken, New Jersey, 2014, Chap. 2, 17.
Application
Deprotection of BOM Groups using Benzenethiol and Boron Trifluoride

References
- Protection for the Hydroxyl Group, Including 1,2- and 1,3-Diols
- P. G. M. Wuts, in Greene’s Protective Groups in Organic Synthesis, 5th ed., ed. by P. G. M. Wuts, John Wiley & Sons, Inc., Hoboken, New Jersey, 2014, Chap. 2, 17.
Application
BF3·Et2O Functioning as a Fluorine Source in an PhIO mediated Intermolecular Aminofluorination

Typical Procedure: To a solution of N-(but-3-en-1-yl)-4-methylbenzenesulfonamide (45 mg, 0.2 mmol) in CH2Cl2 (6 mL) in a flask are added BF3·Et2O (28.2 mg, 0.2 mmol) and PhIO (88 mg, 0.4 mmol). The reaction is stirred at room temperature for 4 h. The resulting mixture is diluted with ethyl acetate (50 mL). Then, the mixture is washed with sat. aqueous NaHCO3 (5 mL) and Na2S2O3 (5 mL). The separated aqueous phase is extracted with EtOAc (2×10 mL). The combined organic layer is washed with brine (10 mL), dried over MgSO4 and concentrated in vacuo to afford the crude product which is purified by flash column chromatography (Rf = 0.12, petroleum ether/EtOAc = 9/1) to give 3-fluoro-1-tosylpyrrolidine as a colorless solid (39 mg, 80% yield).
References
Application
Stereoselective ring-opening hydrofluorination of aryl epoxides

Typical procedure: (RS,RS)-2-Methyl-3-phenyloxirane (4.5 g), BF3· OEt2 (1.38 mL) and CH2Cl2 (134 mL) were reacted at 20 °C for 5 min. Saturated aqueous NaHCO3 was then added and the mixture was stirred vigorously until both layers became clear. The layers were separated and the organic phase was washed with sat. aq. NaHCO3. The combined aqueous phases were extracted twice with CH2Cl2 and the combined organic extracts were dried and concentrated in vacuo. The residue was purified by flash column chromatography (gradient elution, 5 % to 40 % EtOAc in 30~40 °C petrol) gave (RS,RS)-1-fluoro-1-phenylpropane-2-ol as a colorless oil (4.31 g, 83 %, more than 99:1 dr).
References
PubMed Literature
Please visit the Pubchem page at this link.
Articles/Brochures
TCIMAIL
[Product Highlights] Hypervalent Iodine-Catalyzed Balz–Schiemann Fluorination[Research Articles] BF3·Et2O Functioning as a Fluorine Source in an PhIO mediated Intermolecular Aminofluorination
[Research Articles] Stereoselective Ring-Opening Hydrofluorination of Aryl Epoxides
Product Documents (Note: Some products will not have analytical charts available.)
Safety Data Sheet (SDS)
Please select Language.
The requested SDS is not available.
Please Contact Us for more information.
Specifications
C of A & Other Certificates
Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
Sample C of A
This is a sample C of A and may not represent a recently manufactured lot of the product.
A sample C of A for this product is not available at this time.
Analytical Charts
Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
The requested analytical chart is not available. Sorry for the inconvenience.









![Hydrogen Chloride - Methanol Reagent (5-10%) [for Esterification] Hydrogen Chloride - Methanol Reagent (5-10%) [for Esterification]](/_ui/responsive/theme-tci/images/missing_product_srp.jpg)