新客好礼,乐享不停 | 盲盒好礼,精彩继续 | 轻松扫码查看产品文档 | TCIMAIL No.199 已上新 | TCI试剂——品质可靠,值得信赖
订购方法?联系方式:021-67121386 / Sales-CN@TCIchemicals.com
Maximum quantity allowed is 999
请选择数量
CAS RN: 719-98-2 | 产品编码: T3143
N-(Trifluoromethylthio)phthalimide

* 点击“查询”可查看预计发货日期,仅供参考。
* 无具体发货日期的情况,如:显示“8个工作日后发货”,将在您订购日起的8个工作日后发货。
* 我们将以最优方式从上海/天津两大仓库发货。国内库存不足,需两周左右向日本总部调货。
* 对于可分装产品,11:30前的订单,当天发货;11:30后的订单,隔天发货。
* 如需大包装,请点击“大包装询价”按钮(对于某些产品我们无法提供大包装)。
* TCI会经常复审储藏条件以对其进行优化,请以在线目录为准,敬请留意。
* 更多信息,请联系营业部:021-67121386 / Sales-CN@TCIchemicals.com 。任何货期、规格或包装方面的需求,请联系我们 。
* 无具体发货日期的情况,如:显示“8个工作日后发货”,将在您订购日起的8个工作日后发货。
* 我们将以最优方式从上海/天津两大仓库发货。国内库存不足,需两周左右向日本总部调货。
* 对于可分装产品,11:30前的订单,当天发货;11:30后的订单,隔天发货。
* 如需大包装,请点击“大包装询价”按钮(对于某些产品我们无法提供大包装)。
* TCI会经常复审储藏条件以对其进行优化,请以在线目录为准,敬请留意。
* 更多信息,请联系营业部:021-67121386 / Sales-CN@TCIchemicals.com 。任何货期、规格或包装方面的需求,请联系我们 。
| 产品编码 | T3143 |
纯度/分析方法 
|
>98.0%(GC) |
| 分子式/分子量 | C__9H__4F__3NO__2S = 247.19 |
| 外观与形状(20°C) | 固体 |
储存温度 
|
室温 (15°C以下阴凉干燥处) |
| 储存在惰性气体下 | 存放于惰性气体之中 |
| 应避免的情况 | 空气 |
包装和容器 
|
1G-带有塑料内管的玻璃瓶 (查看图片) |
| CAS RN | 719-98-2 |
| Reaxys-RN | 1464619 |
| PubChem物质ID | 253662481 |
技术规格
| Appearance | White to Almost white powder to crystal |
| Purity(GC) | min. 98.0 % |
| Melting point | 111.0 to 115.0 °C |
物性(参考值)
| 熔点 | 113 °C |
GHS
| 象形图 |

|
| 信号词 | 警告 |
| 危险性说明 | H315 : 造成皮肤刺激。 H319 : 造成严重眼刺激。 |
| 防范说明 | P264 : 作业后彻底清洗皮肤。 P280 : 戴防护手套/戴防护眼罩/戴防护面具。 P302 + P352 : 如皮肤沾染:用水充分清洗。 P337 + P313 : 如仍觉眼刺激:求医/就诊。 P305 + P351 + P338 : 如进入眼睛:用水小心冲洗几分钟。如戴隐形眼镜并可方便地取出,取出隐形眼镜。继续冲洗。 P362+P364 : 脱掉沾污的衣服,清洗后方可重新使用。 P332 + P313 : 如发生皮肤刺激:求医/就诊。 |
相关法规
| 新化学物质备案回执号 | B1A232216157 |
运输信息
| 监管条件代码(*) |
应用
Catalytic Trifluoromethylthiolation Using N-(Trifluoromethylthio)phthalimide
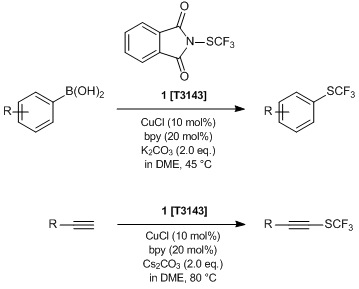
Typical Procedure (Reaction of 1 with boronic acids)3):
CuCl (4.0 mg, 0.04 mmol, 10 mol%), dry boronic acid (0.4 mmol, 1.0 eq.), dry K2CO3 (111 mg, 0.8 mmol, 2.0 eq.), 2,2’-bipyridine (12.5 mg, 0.08 mmol, 20 mol%) and N-(trifluoromethylthio)phthalimide (103.8 mg, 0.42 mmol, 1.05 eq.) are placed into an oven-dried Schlenk tube equipped with a stirring bar. The tube is sealed with a rubber stopper, evacuated and back-filled with dry argon (three times). Dry, freshly distilled and degassed DME is added by a syringe and the reaction mixture is stirred at 45 °C for 18 h. The reaction mixture is cooled to room temperature, diluted with diethyl ether (5 mL), and filtered through a short plug of silica, eluting with additional diethyl ether (50 mL). The filtrate is concentrated; pentane (10 mL) is added and concentrated again for removing residual DME. The resulting residue is purified by chromatography (pentane―diethyl ether) to provide the desired product.
CuCl (4.0 mg, 0.04 mmol, 10 mol%), dry boronic acid (0.4 mmol, 1.0 eq.), dry K2CO3 (111 mg, 0.8 mmol, 2.0 eq.), 2,2’-bipyridine (12.5 mg, 0.08 mmol, 20 mol%) and N-(trifluoromethylthio)phthalimide (103.8 mg, 0.42 mmol, 1.05 eq.) are placed into an oven-dried Schlenk tube equipped with a stirring bar. The tube is sealed with a rubber stopper, evacuated and back-filled with dry argon (three times). Dry, freshly distilled and degassed DME is added by a syringe and the reaction mixture is stirred at 45 °C for 18 h. The reaction mixture is cooled to room temperature, diluted with diethyl ether (5 mL), and filtered through a short plug of silica, eluting with additional diethyl ether (50 mL). The filtrate is concentrated; pentane (10 mL) is added and concentrated again for removing residual DME. The resulting residue is purified by chromatography (pentane―diethyl ether) to provide the desired product.
References
- 1)Trifluoromethylsulfenylation of masked carbonyl compounds
- 2)N-Trifluoromethylthiophthalimide: a stable electrophilic SCF3-reagent and its application in the catalytic asymmetric trifluoromethylsulfenylation
- 3)Direct catalytic trifluoromethylthiolation of boronic acids and alkynes employing electrophilic shelf-stable N-(trifluoromethylthio)phthalimide
PubMed Literature
文档
产品文档 (部分产品的分析图谱无法提供,敬请谅解。)






