Maximum quantity allowed is 999
CAS RN: 133745-75-2 | 产品编码: F0335
N-Fluorobenzenesulfonimide [Fluorinating Reagent]
![N-Fluorobenzenesulfonimide [Fluorinating Reagent] No-Image](/medias/F0335.jpg?context=bWFzdGVyfHJvb3R8NDYzMDV8aW1hZ2UvanBlZ3xhREZrTDJnMFlTODRPVEl4TmpnNU16VTRNelkyTDBZd016TTFMbXB3Wnd8NDJkMWVkY2MxYjM2N2FjOWNlNDk2ZjkxOWJhNjljYWEwNmQzMzY5ZTBhYWFlMDYyOGMxM2M5NmIzNjA3MmVhOQ)
* 无具体发货日期的情况,如:显示“8个工作日后发货”,将在您订购日起的8个工作日后发货。
* 我们将以最优方式从上海/天津两大仓库发货。国内库存不足,需两周左右向日本总部调货。
* 对于可分装产品,11:30前的订单,当天发货;11:30后的订单,隔天发货。
* 如需大包装,请点击“大包装询价”按钮(对于某些产品我们无法提供大包装)。
* TCI会经常复审储藏条件以对其进行优化,请以在线目录为准,敬请留意。
* 更多信息,请联系营业部:021-67121386 / Sales-CN@TCIchemicals.com 。任何货期、规格或包装方面的需求,请联系我们 。
| 产品编码 | F0335 |
| 纯度/分析方法 | >98.0%(T)(HPLC) |
| 分子式/分子量 | C__1__2H__1__0FNO__4S__2 = 315.33 |
| 外观与形状(20°C) | 固体 |
| 储存温度 | 室温 (15°C以下阴凉干燥处) |
| 包装和容器 | 1G-带有塑料内管的玻璃瓶 (查看图片) |
| CAS RN | 133745-75-2 |
| Reaxys-RN | 5348902 |
| PubChem物质ID | 87570107 |
| SDBS (AIST Spectral DB) | 19601 |
| MDL编号 | MFCD00144885 |
| Appearance | White to Light yellow powder to crystal |
| Purity(HPLC) | min. 98.0 area% |
| Purity(Iodometric Titration) | min. 98.0 % |
| Melting point | 115.0 to 119.0 °C |
| 熔点 | 115 °C |
| 象形图 |


|
| 信号词 | 警告 |
| 危险性说明 | H315 : 造成皮肤刺激。 H319 : 造成严重眼刺激。 H341 : 怀疑可造成遗传性缺陷。 |
| 防范说明 | P501 : 将内装物/容器送到批准的废物处理厂处理。 P202 : 在阅读并明了所有安全措施前切勿搬动。 P201 : 使用前取得专用说明。 P264 : 作业后彻底清洗皮肤。 P280 : 戴防护手套/穿防护服/戴防护眼罩/戴防护面具。 P302 + P352 : 如皮肤沾染:用水充分清洗。 P308 + P313 : 如接触到或有疑虑:求医/就诊。 P337 + P313 : 如仍觉眼刺激:求医/就诊。 P305 + P351 + P338 : 如进入眼睛:用水小心冲洗几分钟。如戴隐形眼镜并可方便地取出,取出隐形眼镜。继续冲洗。 P362+P364 : 脱掉沾污的衣服,清洗后方可重新使用。 P332 + P313 : 如发生皮肤刺激:求医/就诊。 P405 : 存放处须加锁。 |
| 新化学物质备案回执号 | B1A232216039 |
| 监管条件代码(*) |

-
Used Chemicals
-
Procedure
-
To a solution of 4-bromobiphenyl (1.17 g, 5.00 mmol), DABSO (721 mg, 3.00 mmol), and PdCl2(Amphos)2 (177 mg, 0.25 mmol) in anhydrous isopropyl alcohol (20 mL) was added triethylamine (2.1 mL, 15 mmol) at room temperature and the mixture was stirred at 75 °C for 23 hours. The suspension was cooled at room temperature and NFSI (2.36 g, 7.5 mmol) was added and the reaction mixture stirred for 3 hours until completion. The solvent was removed under reduced pressure. The residue was diluted with ethyl acetate (20 mL) and filtrated through celite pad. The filtrate was washed with saturated aqueous Na2S2O3 solution (20 mL) and brine (20 mL), dried over sodium sulfate and filtered. The solvent was removed under reduced pressure and the residue was purified by medium pressure preparative chromatography (ethyl acetate:hexane = 2:98 - 5:95 on silica gel) to give compound 1 as a white solid (928 mg, 79%).
-
Experimenter’s Comments
-
The reaction mixture was monitored by TLC (ethyl acetate:hexane = 1:9, Rf = 0.57) and UPLC-MS.
-
Analytical Data
-
Compound 1
1H NMR (400 MHz, CDCl3); δ 8.12–8.04 (m, 2H), 7.86–7.80 (m, 2H), 7.67–7.58 (m, 2H), 7.44-7.55 (m, 3H).
-
Lead Reference
-
- One-pot palladium-catalyzed synthesis of sulfonyl fluorides from aryl bromides
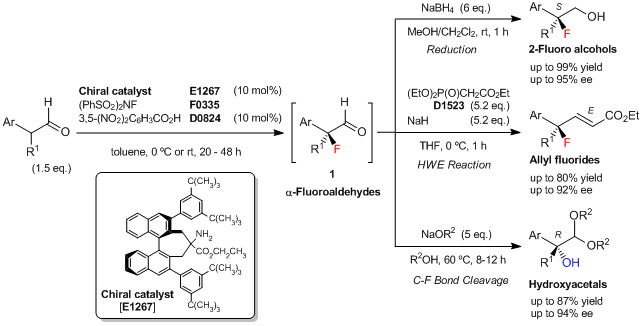
To a solution of the chiral amine catalyst [E1267] (20 mg, 0.026 mmol, 10 mol%) in toluene (0.54 mL) is added 3,5-dinitrobenzoic acid (5.5 mg, 0.026 mmol, 10 mol%), 2-phenylpropionaldehyde (52 mg, 0.39 mmol, 1.5 eq.), and N-fluorobenzenesulfonimide (NFSI) (0.26 mmol, 82 mg, 1 eq.) at 0 °C. The reaction mixture is stirred at 0 °C for 48 h, then poured into CH3OH/CH2Cl2 (1:4, 1.3 mL) at 0 °C. To this solution, NaBH4 (1.6 mmol, 6 eq.) is added, and the mixture is stirred at room temperature for 1 h. The reaction is quenched with saturated aq.NH4Cl, and the mixture is extracted with Et2O. The organic layer is dried over Na2SO4, and concentrated. The residue is purified by silica gel chromatography (eluent: hexane/ethyl acetate = 3/1) to give (S)-2-fluoro-2-phenylpropan-1-ol (34.5 mg, 86% yield based on NFSI, 95% ee) as a white solid.
References

In a dry 25 mL round-bottom flask, thiophene (0.5 mmol, 1 eq.), NFSI (0.6 mmol, 1.2 eq.) and CuI (5 mol%) are dissolved in DCE (3 mL), the resulting mixture is put into a preheated oil bath (60 °C) for 8 h. The solution is then cooled to room temperature, evaporated under reduced pressure, diluted with ethyl acetate, washed with a saturated aqueous solution of NaCl, dried over Na2SO4, filtered and evaporated under reduced pressure. The crude product is purified by silica gel column chromatography to afford the corresponding product (166.7 mg, Y. 88 %).
References

Reference
文章/手册
[研究论文] 铜催化杂环化合物和N-氟代苯磺酰亚胺的直接酰胺化反应
[TCI应用实例] 使用DABSO和NFSI对芳基溴化物进行一锅法钯催化的氟磺酰化反应
化学品安全说明书(SDS)
如需更多帮助,请联系我 们。
技术规格
CoA及其他文档
示例 CoA
目前没有该产品的 CoA 示例。
分析图谱
很抱歉,您搜索的分析图谱无法提供。



![N-Fluorobenzenesulfonimide [Fluorinating Reagent] N-Fluorobenzenesulfonimide [Fluorinating Reagent]](/_ui/responsive/theme-tci/images/missing_product_zoom.jpg)


