网站维护通知 (2025年11月1日11:00~16:30):由于系统维护,网站暂时不可用,敬请谅解!
联系方式:021-67121386 / Sales-CN@TCIchemicals.com
Maximum quantity allowed is 999
请选择数量
氟化反应 [合成试剂]
The introduction of fluorine into bioactive molecules often results in significant changes in their chemical, physical, and biological properties. Fluorine uniquely affects the property of organic molecules due to the fluorine atom’s blocking effect in metabolic transformations and mimicking of enzyme substrates, and then increases the molecular lipophilicity to enhance bioavailability. Approximately 30% of all agrochemicals and 20% of all pharmaceuticals contain fluorine.1) HMG-CoA reductase inhibitor fluorostatin (atorvastatin), antibiotic fluoroquinolone (levofloxacin) , and antitumor fluoronucleoside (tegafur) are successful examples of the introduction of fluorine into organic molecules (Figure). Therefore, fluorinating reagents are useful tools for the synthesis of pharmaceutical and agricultural compounds.

Figure. Fluorinated bioactive compounds
Since there are few naturally occurring fluorine containing compounds, it is necessary to fluorinate organic compounds at a certain stage of the syntheses. Though fluorine gas and hydrogen fluoride are used as fluorin e sources, they are very toxic and corrosive and require special equipment and techniques for handling them. Therefore, alternate fluorinating agents have been developed, which are used in laboratories to easily introduce a fluorine atom at selected positions of the compounds. The fluorinating agents are roughly classified into two categories: nucleophilic and electrophilic. Nucleophilic fluorinating agents are those where the fluoride anion serves as a reaction active species. Electrophilic fluorinating agents are those where the electron-deficient fluorine atom serves as a reaction active species.
1. Nucleophilic Fluorinating Agents
The most basic nucleophilic fluorinating agent is hydrogen fluoride which is used in large quantity for the industrial production of fluorinated compounds. Hydrogen fluoride (HF), however, is scarcely used in laboratories due to its toxic and corrosive properties and its low reactivity resulting from high H-F bond energy. Nucleophilic fluorinating agents such as KF, CsF, and Bu4N·F are readily available.
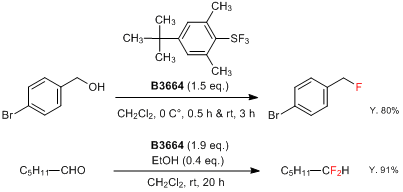
4-tert-Butyl-2,6-dimethylphenylsulfur trifluoride (= FLUOLEAD™) (Product No. B3664), which was first reported by Umemoto, is a novel nucleophilic fluorinating reagent.2) FLUOLEAD is a white crystalline solid with high thermal stability, which has a 232 °C thermal decomposition temperature (by DSC analysis). Differing from other existing fluorinating reagents, such as DAST, FLUOLEAD has less fuming character. And the reactivity of FLUOLEAD to water is slow, which makes it easier to handle in open air. Fluorinations of a hydroxyl or carbonyl group by FLUOLEAD afford the corresponding fluorinated compounds in good yields under a wide range of conditions (0 to 100 °C).
4-tert-Butyl-2,6-dimethylphenylsulfur trifluoride (= FLUOLEAD™) (Product No. B3664), which was first reported by Umemoto, is a novel nucleophilic fluorinating reagent.2) FLUOLEAD is a white crystalline solid with high thermal stability, which has a 232 °C thermal decomposition temperature (by DSC analysis). Differing from other existing fluorinating reagents, such as DAST, FLUOLEAD has less fuming character. And the reactivity of FLUOLEAD to water is slow, which makes it easier to handle in open air. Fluorinations of a hydroxyl or carbonyl group by FLUOLEAD afford the corresponding fluorinated compounds in good yields under a wide range of conditions (0 to 100 °C).
2. Electrophilic Fluorinating Agents
The basic agent among electrophilic fluorinating agents is fluorine gas, which is not suitable for partial fluorination due to its vigorous reactivity and strong toxicity.
N-Fluoro-N'-(chloromethyl)triethylenediamine bis(tetrafluoroborate) (Product No. F0358) is an easily handled powder and versatile electrophilic fluorinating agent for enols, silyl enol ethers, alkenes, stabilized carbanions, aromatic compounds, organosulfur compounds, Grignard reagents, etc.3)
N-Fluoro-N'-(chloromethyl)triethylenediamine bis(tetrafluoroborate) (Product No. F0358) is an easily handled powder and versatile electrophilic fluorinating agent for enols, silyl enol ethers, alkenes, stabilized carbanions, aromatic compounds, organosulfur compounds, Grignard reagents, etc.3)

Topics
- Electrophilic Trifluoromethylating Agent, Umemoto Reagent IV / Electrophilic Fluorinating Agent, NFBB
- TCI Practical Example: The Deoxyfluorination of Alcohol Using a Photoredox Catalyst
- PhenoFluor™ Mix and AlkylFluor™: Stable Reagents for Deoxyfluorinations
- Hypervalent Iodine-Catalyzed Balz–Schiemann Fluorination
- An Air- and Moisture- stable Electrophilic Fluorinating Reagent
- BF3·Et2O Functioning as a Fluorine Source in an PhIO mediated Intermolecular Aminofluorination
- Metal-Free Fluorination of C(sp3)-H Bonds using a Catalytic N-Oxyl Radical
- Stable Fluorinating Reagent with Ease of Handling (FLUOLEAD™)
References
- 主页
- 产品
- 化学
- 合成试剂
- 氟化试剂 [合成试剂]
- 氟化反应 [合成试剂]

