Published TCIMAIL newest issue No.196
Maximum quantity allowed is 999
Merci de sélectionner la quantité
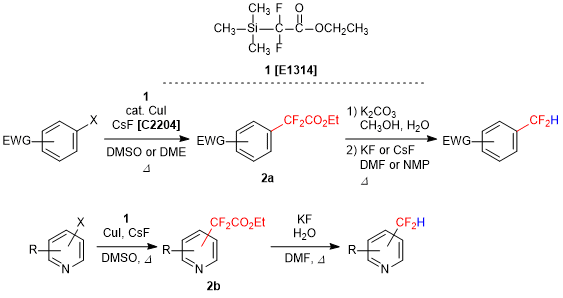
Difluoroacetate Derivative for the Synthesis of Difluoromethylaryls
Ethyl 2,2-difluoro-2-(trimethylsilyl)acetate (1) is utilized to introduce a difluoromethyl group in aromatic ring compounds. In the presence of copper(I) iodide and cesium fluoride, (hetero)aryl halides react with 1 to give 2-aryldifluoromethylacetate esters 2a and 2b. Both 2a and 2b can be subsequently converted into difluoromethyl aryl compounds via hydrolysis and decarboxylation.1) Furthermore, pyridine derivative 2b affords corresponding difluoromethyl compounds in a single operation upon treatment with potassium fluoride in water.2) Difluoromethyl group has a high lipophilicity and an electro-withdrawing power, and also behaves as a hydrogen bond donor, so it is focused on the researches of pharmaceuticals and agrochemicals. As a result, 1 is anticipated as a promising compound for the introduction of difluoromethyl group.
Related Products
References
- 1) A New Method for Aromatic Difluoromethylation: Copper-Catalyzed Cross-Coupling and Decarboxylation Sequence from Aryl Iodides
- 2) An Efficient Route to Difluoromethylated Pyridines

