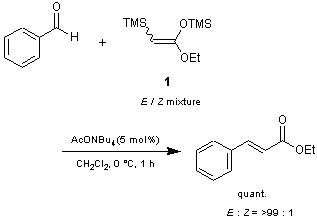
Mukaiyama and Michida have reported that E/Z mixtures of ketene acetals 1 and 2 react with various kinds of aldehydes in the presence of a catalytic amount of acetate salts to give α,β-unsaturated esters in high yields with excellent stereoselectivities.1) This procedure has some advantages over conventional methods such as the Peterson reaction and the Horner-Wadsworth-Emmons (HWE) reaction. For example:
1) Catalytic reaction (with no need of equimolar amount of base); 2) Co-product (TMS2O) can be removed easily by evaporation; 3) High stereoselectivity is obtained by the proper choice of the counter cation of the catalyst.
Sign up for free shipping on all website orders, no minimum required, and get exclusive coupons!
Users can now ask questions to the AI chatbot by clicking the chatbot icon in the bottom-right corner of the website.