Sign up for free shipping on all website orders, no minimum required, and get exclusive coupons!
Users can now ask questions to the AI chatbot by clicking the chatbot icon in the bottom-right corner of the website.
Maximum quantity allowed is 999
| Taille | Prix unitaire | Philadelphia, PA | Portland, OR | Japon * | Quantité |
Informations d'expédition

|
|---|---|---|---|---|---|---|
| 250MG |
$85.00
|
9 | 1 | Contactez-nous |
|
|
| 1G |
$265.00
|
9 | 27 | 16 |
|
* Les articles en stock local sont généralement expédiés le jour même de la commande. Les articles en stock au Japon peuvent être expédiés depuis un entrepôt américain dans les deux semaines suivant la commande. Pour obtenir une estimation plus précise des délais d’expédition, veuillez consulter l’outil de simulation d’expédition. Remarque : Sont exclus les articles réglementés ainsi que les articles nécessitant un envoi sous glace.
*Les articles en stock localement sont expédiés dans un délai de 1 à 2 jours. Les articles en stock au Japon peuvent être expédiés depuis un entrepôt aux Etats-Unis dans un délai de 2 semaines. Veuillez contacter TCI pour connaître les délais de livraison des articles qui ne sont pas en stock. Cela ne concerne par les articles réglementés et les articles expédiés sur carboglace.
*TCI révise régulièrement les conditions de stockage afin de les optimiser. Veuillez noter que les informations les plus récentes sur la température de stockage des produits sont disponibles sur notre site web.
| Numéro de produit | B3161 |
Pureté / Méthode d'analyse 
|
>98.0%(T) |
| Formule moléculaire / poids moléculaire | C__2__4H__5__4P__2Pd = 511.06 |
| Etat physique (20 ° C) | Solid |
Condition de stockage 
|
Frozen (<0°C) |
| Stocker sous gaz inerte | Store under inert gas |
| Condition à éviter | Air Sensitive,Heat Sensitive |
Emballage Et Conteneur 
|
1G-Glass Bottle with Plastic Insert (Voir l'image) , 250MG-Glass Bottle with Plastic Insert (Voir l'image) |
| CAS RN | 53199-31-8 |
| Numéro de registre de Reaxys | 14300595 |
| Identifiant de la substance PubChem | 87560386 |
| Numéro MDL | MFCD03094580 |
| Appearance | White to Yellow to Orange powder to crystal |
| Purity(Chelometric Titration) | min. 98.0 % |
| Numéro du SH (importation) (TCI-A) | 2942.00.5000 |
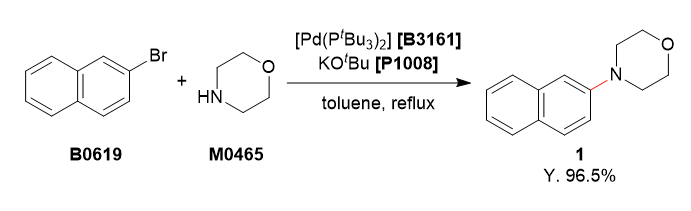
To a 4-necked 200 mL flask was charged with 2-bromonaphthalene (5.20 g, 25.1 mmol, 1.0 equiv.), morpholine (3.27 mL, 37.5 mmol, 1.5 equiv.) and degassed toluene (75 mL). To this solution was added bis(tri-tert-butylphosphine)palladium(0) (256 mg, 0.501 mmol, 2.0 mol%) and potassium tert-butoxide (4.21 g, 37.5 mmol, 1.5 equiv.). The reaction mixture was refluxed for 3 h under argon atmosphere. The reaction mixture was cooled to room temperature and washed with water (50 mL). The organic layer was separated and the aqueous layer was extracted with ethyl acetate (50 mL). The combined organic layers were washed with brine (50 mL), dried over Na2SO4 and filtered. The solvent was removed under reduced pressure. The residue was purified by column chromatography (eluent: hexane/ethyl acetate, 85/15→70/30) to obtain 1 as a white solid (5.17 g, 96.5%).
The reaction mixture was monitored by TLC (hexane/ethyl acetate = 9/1, Rf = 0.70).
1H NMR (400 MHz, CDCl3); δ 7.76–7.69 (m, 3H), 7.42 (t, J = 7.2 Hz, 1H), 7.31 (t, J = 6.8 Hz, 1H), 7.25–7.28 (m, 1H), 7.13 (s, 1H), 3.92 (t, J = 4.8 Hz, 2H), 3.27 (t, J = 4.8 Hz, 2H).
13C NMR (101 MHz, CDCl3); δ 129.0, 127.6, 127.0, 126.5, 123.7, 119.1, 110.3, 67.1, 50.0.
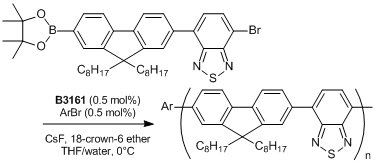

Typical Procedure: An ampoule is charged with the thiocarbamate (0.442 mmol) and anhydrous degassed toluene (4 mL) is added via gastight syringe under nitrogen with stirring. A J. Youngs valve is fitted and the ampoule is placed into an oil bath pre-equilibrated at 100℃. After ca. 5 minutes the valve is removed and Pd(t-Bu3P)2 (2 mol%, 0.00884 mmol) is added as a solid, the valve is then replaced and the reaction mixture heated for 2.5 hours. A sample of the reaction mixture is removed via gastight syringe and found to contain the desired product (>99%).
La FDS demandée n'est pas disponible.
Nous contacter pour plus d'informations.
Un échantillon CoA pour ce produit n'est pas disponible pour le moment.

Le tableau analytique demandé n'est pas disponible. Nous sommes désolés pour ce désagrément.