Published TCIMAIL newest issue No.197
Maximum quantity allowed is 999

The TCO (trans-cyclooctene) moiety is known for its high internal strain on the double bond, facilitating the Strain-Promoted Inverse Electron-Demand Diels-Alder Reaction (SPIEDAC) with tetrazine derivatives. This reaction proceeds selectively even in the presence of various functional groups, making it applicable as a click chemistry tool. Click chemistry utilizing TCO does not require metal catalysts and exhibits rapid reaction rates as its primary feature. This click chemistry meets the criteria for bioorthogonal reactions (fast, selective, biocompatible, metal-free) and finds applications in a wide range of uses such as protein labeling and imaging.1,2,3,4)
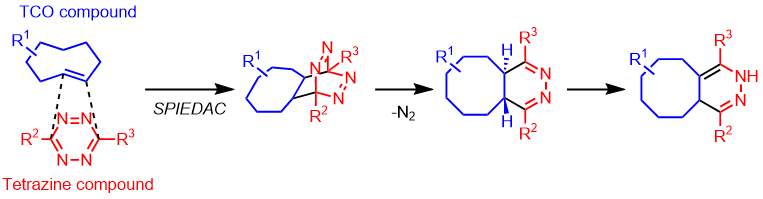
The reaction rate between TCO and tetrazine derivatives is described as [TCO > TCO*] and [axial > equatorial]. However, in biological environments, especially in the presence of thiols, the stability is characterized as [TCO* > TCO].5)
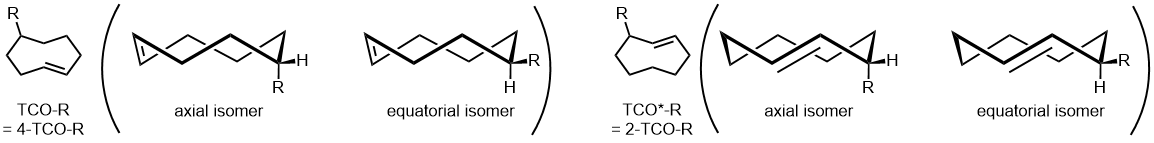
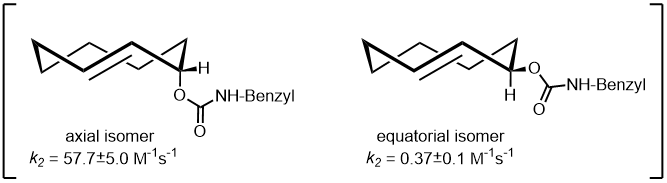
Furthermore, recent reports have indicated that the reaction between 2-TCO derivatives and tetrazine derivatives can exhibit "click-to-release" reactions under metal catalyst-free conditions, showing promise for their role as prodrugs.6,7,8,9)
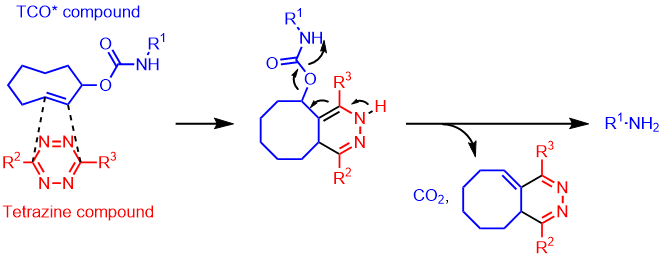
Based on these characteristics, we offer a variety of TCO derivatives useful for synthesis, including 4-nitrophenyl carbonate (NPC) esters, amines elongated with PEG, biotins, DBCOs, NHS esters, maleimide derivatives, and more.
Products
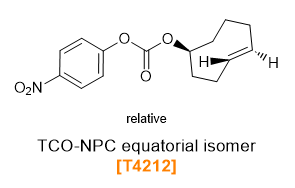
TCO-4-Nitrophenyl Carbonate (NPC) Esters
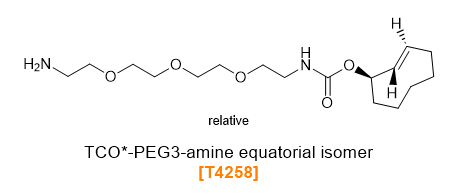
TCO-PEG-Amines
TCO-PEG-DBCOs
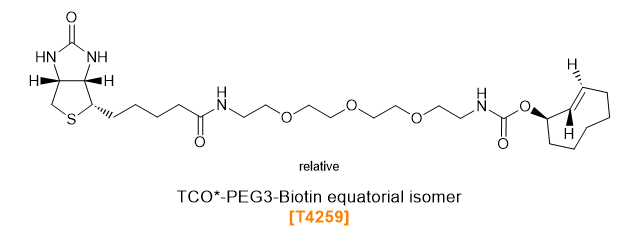
TCO-PEG-Biotins
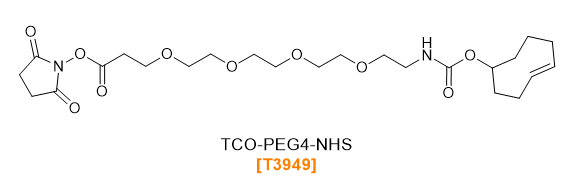
TCO-PEG-NHS Esters
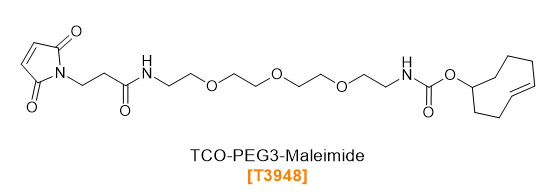
TCO-PEG-Maleimides
TCO-Amino Acid
Related Products
Tetrazines
References
- 1) Tetrazine Ligation: Fast Bioconjugation Based on Inverse-Electron-Demand Diels−Alder Reactivity
- 2) Fast and Sensitive Pretargeted Labeling of Cancer Cells through a Tetrazine/trans-Cyclooctene Cycloaddition
- 3) Bioorthogonal Turn-On Probes for Imaging Small Molecules inside Living Cells
- 4) Inverse electron demand Diels–Alder reactions in chemical biology
- 5) Minimal Tags for Rapid Dual-Color Live-Cell Labeling and Super-Resolution Microscopy
- 6) Click to Release: Instantaneous Doxorubicin Elimination upon Tetrazine Ligation
- 7) In situ activation of a doxorubicin prodrug using imaging-capable nanoparticles
- 8) In Vivo Bioorthogonal Chemistry Enables Local Hydrogel and Systemic Pro-Drug To Treat Soft Tissue Sarcoma
- 9) Optimized Tetrazine Derivatives for Rapid Bioorthogonal Decaging in Living Cells